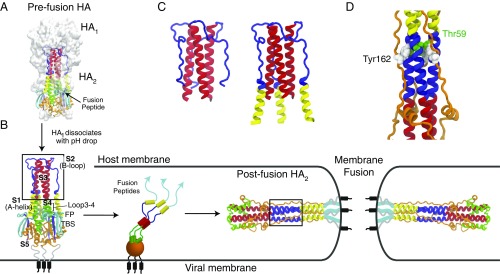
Fig. 1.
The pH-induced HA transition. (A) The prefusion configuration of HA [Protein Data Bank (PDB) ID code 2HMG (2)] including the head domain (white surface representation) and stem domain (colored cartoon representation). (B) The reduction of pH triggers the dissociation of from , which starts an extensive structural rearrangement ending in the postfusion configuration [PDB ID code 1QU1 (4)]. Successful membrane fusion presumably places the FPs and transmembrane helices in the same membrane. Note that remains localized near because of a disulfide bond between regions S5 and TBS (two -strands). The B-loop domain (blue) changes from a random loop to a coiled-coil structure (highlighted by boxes). The central stalk, S3, maintains its coiled-coil structure during the transition. The A-helix domain (yellow) shifts >100 Å from the preconfiguration to postconfiguration. Little is known about intermediate configurations during this transition. (C) The simulation constructs: the entire B loop and S3 (Left); and A helix, B loop, and S3 (Right). (D) The long coiled coil in the postfusion structure is wrapped by S5 (orange domain), which may provide additional stabilization to the coiled-coil configuration. In the crystal structure, Tyr162 is the only residue in S5 that buries deeply into the B loop. A buried water is coordinated between Thr59 and Tyr162.