Evidence-Based Outcomes Article.
Abstract
Background:
The authors investigated aesthetic outcome and patient satisfaction in women who have undergone deep inferior epigastric artery perforator (DIEP) flap reconstruction in the setting of postmastectomy radiotherapy. Patients who underwent DIEP flap reconstruction without postmastectomy radiotherapy were the control group.
Methods:
Participants who had undergone DIEP flap reconstruction between September 1, 2009, and September 1, 2014, were recruited, answered the BREAST-Q, and underwent three-dimensional surface-imaging. A panel assessed the aesthetic outcome by reviewing these images.
Results:
One hundred sixty-seven women participated. Eighty women (48 percent) underwent immediate DIEP flap reconstruction and no postmastectomy radiotherapy; 28 (17 percent) underwent immediate DIEP flap reconstruction with postmastectomy radiotherapy; 38 (23 percent) underwent simple mastectomy, postmastectomy radiotherapy, and DIEP flap reconstruction; and 21 (13 percent) underwent mastectomy with temporizing implant, postmastectomy radiotherapy, and DIEP flap reconstruction. Median satisfaction scores were significantly different among the groups (p < 0.05). Post hoc comparison demonstrated that women who had an immediate DIEP flap reconstruction were significantly less satisfied if they had postmastectomy radiotherapy. In women requiring radiotherapy, those undergoing delayed reconstruction after a simple mastectomy were most satisfied, but there was no significant difference between the immediate DIEP flap and temporizing implant groups. Median panel scores differed among groups, being significantly higher if the immediate reconstruction was not subjected to radiotherapy. There was no significant difference in panel assessment among the three groups of women who had received radiotherapy.
Conclusions:
Patients who avoid having their immediate DIEP flap reconstruction irradiated are more satisfied and have better aesthetic outcome than those who undergo postmastectomy radiotherapy. In women requiring radiotherapy and who wish to have an immediate or “delayed-immediate” reconstruction, there were no significant differences in panel or patient satisfaction. Therefore, immediate DIEP flap reconstruction or mastectomy with temporizing implant then DIEP flap surgery are acceptable treatment pathways in the context of post-mastectomy radiotherapy.
In the United Kingdom, 53 percent of women with symptomatic breast cancer and 27 percent of those with screen-detected breast cancer are treated surgically with mastectomy.1 The immediate breast reconstruction rate was 21 percent at the time of the UK National Mastectomy and Breast Reconstruction Audit in 2007 to 2009.2 In the United States, 35.5 percent of women undergo mastectomy, and rates of immediate breast reconstruction have increased from 11.6 percent in 1998 to 36.4 percent in 2011.3
Adjuvant postmastectomy radiotherapy is offered to women with risk factors such as T3 to T4 disease and/or involved lymph nodes, and its local control and survival benefits are well established.4–8 More recently, oncologic benefit has been demonstrated in women previously considered to be at “intermediate risk” (i.e., patients with N1 disease), such that the criteria for offering postmastectomy radiotherapy are expanding.9,10
The deep inferior epigastric artery perforator (DIEP) flap, which was first popularized by Allen and Treece in 1994,11 has become one of the most reliable and popular methods of autologous breast reconstruction, with published flap failure rates ranging from less than 1 percent to 4 percent.12
Radiotherapy after immediate autologous reconstruction has been thought to have a detrimental impact on aesthetic outcome; therefore, women who are expected to need postmastectomy radiotherapy are often advised to undergo a delayed autologous reconstruction.13 The use of a temporizing implant, also known as “delayed-immediate reconstruction,”14,15 allows the patient to have a chest wall mound while awaiting the planned exchange to autologous reconstruction after radiotherapy and facilitates the preservation of native breast skin. However, this pathway has potentially far-reaching consequences. Not only does the patient require a subsequent operation for the reconstruction but she must live for a considerable amount of time with a flat chest or a tissue expander/implant before the definitive operation. Because of pressures in today’s health care system, the waiting time for the elective reconstruction may be many months.
A systematic review by Kelley et al.12 attempted to address questions related to complications and flap compromise as a result of radiation delivery before or after autologous breast reconstruction. The review analyzed 20 articles with over 1500 flap reconstructions. No significant differences in measurable postoperative complications including total flap loss, wound healing complications, infection, hematoma, seroma, and fat necrosis were found when comparing irradiated versus unirradiated reconstructions. However, differences in cosmetic outcome and patient satisfaction between groups were not addressed.
The aim of our study was to investigate aesthetic outcome and patient satisfaction in women who have undergone DIEP flap reconstruction in the setting of postmastectomy radiotherapy. To achieve this, we compared patients undergoing immediate DIEP flap reconstruction and then radiotherapy with women who had radiotherapy after mastectomy (with or without temporizing implant) and a delayed DIEP flap. We also studied patients who underwent immediate DIEP flap reconstruction without radiotherapy as a control group.
PATIENTS AND METHODS
Recruitment
Regional ethical committee approval was obtained and registered. In January of 2016, patients who had undergone DIEP flap reconstruction (immediate or delayed) between October 1, 2009, and October 1, 2014, were identified. Exclusion criteria included the following:
Bilateral DIEP flap reconstruction.
Delayed reconstruction without postmastectomy radiotherapy.
Subsequent diagnosis of local recurrence, contralateral breast cancer, or metastatic disease.
Subsequent death.
Less than 1 year after the end of oncologic treatment.
Inability to answer the questionnaire or living outside the United Kingdom.
DIEP flap for chest wall resurfacing rather than breast reconstruction.
DIEP flap for nonbreast cancer abnormality (e.g., sarcoma).
DIEP flap for cosmetic failure of other reconstruction/breast conservation (e.g., poor long-term result after latissimus dorsi reconstruction or breast conservation).
“Salvage” DIEP flap; that is, women in whom a definitive implant-based reconstruction was planned but, because of urgent complications (e.g., infection or extrusion) or severe pain/cosmetic failure caused by capsule formation, the implant was removed and replaced with a DIEP flap.
Patients were divided into four groups:
A. Skin-sparing mastectomy with immediate DIEP flap reconstruction without postmastectomy radiotherapy (control group).
B. Skin-sparing mastectomy with immediate DIEP flap reconstruction and postmastectomy radiotherapy.
C. Simple mastectomy (no skin preservation), postmastectomy radiotherapy, and delayed DIEP flap reconstruction.
D. Skin-sparing mastectomy and preservation of skin flaps with temporizing implant, postmastectomy radiotherapy, and later DIEP flap reconstruction.
This cohort largely predates the use of acellular dermal matrices in our unit. If autologous reconstruction was the patient’s preference and radiotherapy was anticipated, our usual practice was to offer a temporizing implant (in the form of a subpectoral expandable implant) and replacement with a DIEP flap 12 months or more after postmastectomy radiotherapy. Toward the end of the inclusion period, we began to offer immediate DIEP flap reconstruction to selected women who were likely to require radiotherapy because it was anecdotally noted that women who had undergone immediate DIEP flap reconstruction and then unexpectedly needed radiotherapy had a good cosmetic outcome. Patients were counseled before surgery of the potential effect of radiotherapy to an immediate DIEP flap reconstruction, and those who were willing to accept this rather than a delayed approach were considered for an immediate reconstruction. In particular, patients with large breasts were felt to have little to gain from a temporizing implant approach because of the resulting asymmetry after a delayed DIEP flap and requirement for a contralateral symmetrizing procedure; therefore, these patients began to be considered for immediate reconstruction despite the need for radiotherapy. In the simple mastectomy group, the lower pole skin is usually found to constrict the potential envelope and is excised, to be replaced by more compliant skin from the abdomen.
Postmastectomy radiotherapy was delivered to the chest wall with or without the reconstructed breast with or without the supraclavicular fossa to a dose of 40 Gy in 15 fractions over 3 weeks. A simple forward-planned intensity-modulated field-based technique was used in which the chest wall was treated with tangential fields (with dose homogenization using a step-and-shoot technique) and the supraclavicular fossa with a matched anterior field. Only in cases with involved anterior margins or inflammatory breast cancer was skin bolus (1 cm thickness) applied to the chest wall with or without the reconstructed breast for all 15 fractions. In all other cases, bolus was not applied.
Eligible patients were invited to participate by letter. Participants underwent medical photography using the Vectra XT (Canfield Scientific, Fairfield, N.J.) three-dimensional surface imaging system and completed the BREAST-Q reconstruction module.16–18 The clinicopathologic data set was collected independently from the patient satisfaction and panel assessment results. Complication data were collected only for breast complications (not donor site).
Patient-Reported Satisfaction
The BREAST-Q is a validated patient-reported outcome measurement tool that is specifically designed to measure satisfaction and quality of life in women who have undergone breast reconstruction. For this study, the first domain, “Satisfaction with Breasts,” was used. Within this domain are 16 subquestions answered according to a four-point Likert scale. The total score is transformed into a score out of 100. The higher the score, the more satisfied the patient reports to be.
Surgeon-Reported Aesthetic Outcome
A panel assessment of aesthetic outcome was undertaken. The three-dimensional image was rotated so it could be viewed from all angles (Fig. 1). The 10-point score by Visser et al.19 was used, as it was rated best in a review article comparing panel assessments for breast reconstruction using the modified Scientific Advisory Committee’s Medical Outcomes Trust Criteria20 and has been used in several other published studies.21–29 In this scoring system, volume, shape, symmetry, scarring, and nipple-areola complex are scored from 1 to 5, and a global score from 1 to 10 is assigned to each patient, whereby a higher total score indicates a better aesthetic outcome. The panel consisted of two plastic surgeons and two breast surgeons. The panel members scored independently, and the median score was used in the analysis. The panel members were blinded to which treatment arms the patients were in.
Fig. 1.
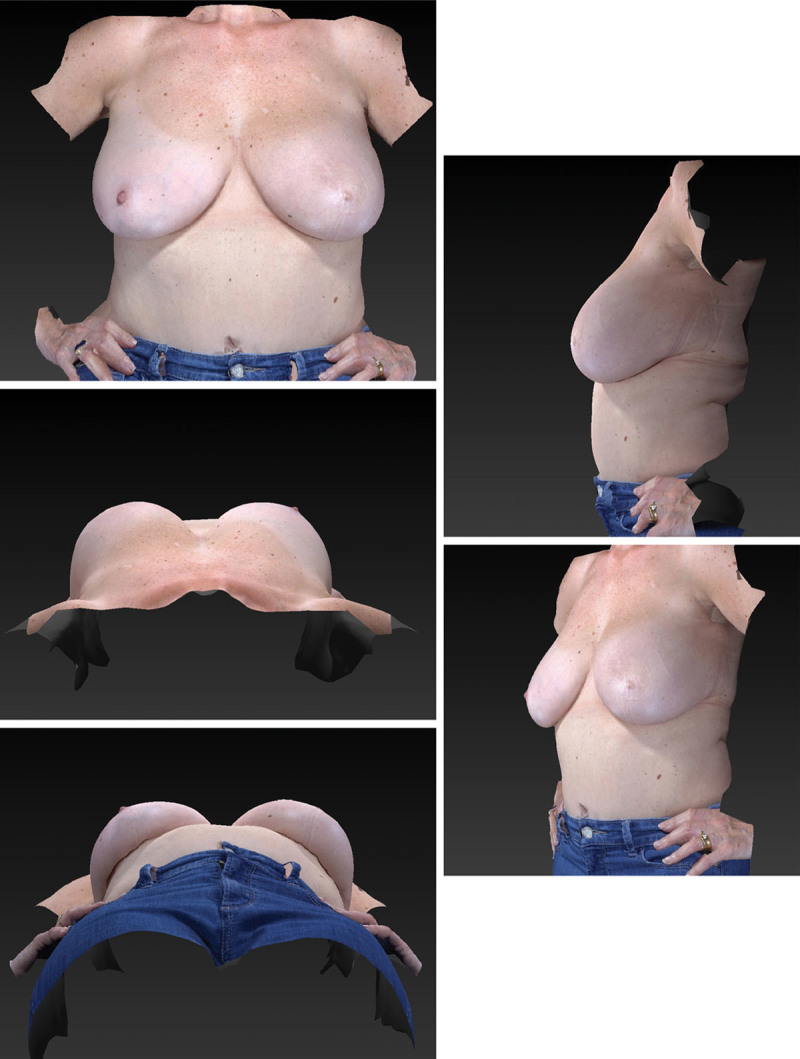
Images produced using the three-dimensional imaging system include (above, left) anteroposterior, (above, right) lateral, (center, left) craniocaudal, (below, right) oblique, and (below, left) caudocranial.
Statistical Analysis
Demographics were presented as descriptive statistics, and any quantitative variables were presented as mean and standard deviation or median and interquartile range, as appropriate after testing for normality. Qualitative data were presented as proportions and frequencies, and the chi-square or Fisher’s exact test was used to compare the groups.
A kappa statistic was used to assess the inter rater agreement between the surgeons assessing aesthetic outcome in the panel assessment. A value of 0 indicates no agreement, 0 to 0.20 is slight agreement, 0.21 to 0.40 is fair agreement, 0.41 to 0.60 is moderate agreement, 0.61 to 0.80 is substantial agreement, is 0.81 to 1 is almost perfect agreement.
After testing for normality, the Kruskal-Wallis test was used to assess the relationship of patient satisfaction and panel score of aesthetic outcome with the patient treatment group (1 through 4). The Dunn-Sidak test (a post hoc adjusted pairwise comparison) was used to identify between which pairs of treatment groups the significant differences lay. Any pairwise comparisons with a value of p < 0.05 were considered statistically significant. Analysis was performed using Stata (StataCorp, College Station, Texas).
RESULTS
Between October 1, 2009, and October 1, 2014, 476 women underwent DIEP flap reconstruction. There were no flap losses in the cohort. One hundred seventy-seven women were not eligible because of bilateral surgery (n = 22), delayed reconstruction without postmastectomy radiotherapy (n = 42), less than 1 year after the end of oncologic treatment (n = 5), local recurrence (n = 5), contralateral breast cancer (n = 4), metastatic disease (n = 15), died (n = 12), unable to answer questionnaire or live outside the United Kingdom (n = 40), sarcoma (n = 5), previous latissimus dorsi reconstruction (n = 5), poor result from previous breast conservation therapy (n = 1), chest wall resurfacing (n = 1), or salvage DIEP flap reconstruction (n = 18). It was not possible to contact 101 women and 30 declined to participate. Three women did not attend the arranged appointment. Therefore, in total, 167 women participated in the study (Fig. 2).
Fig. 2.
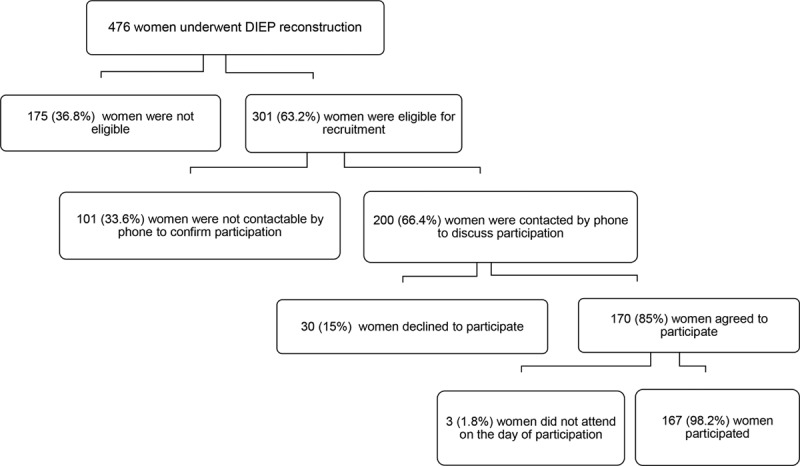
Diagram of recruitment.
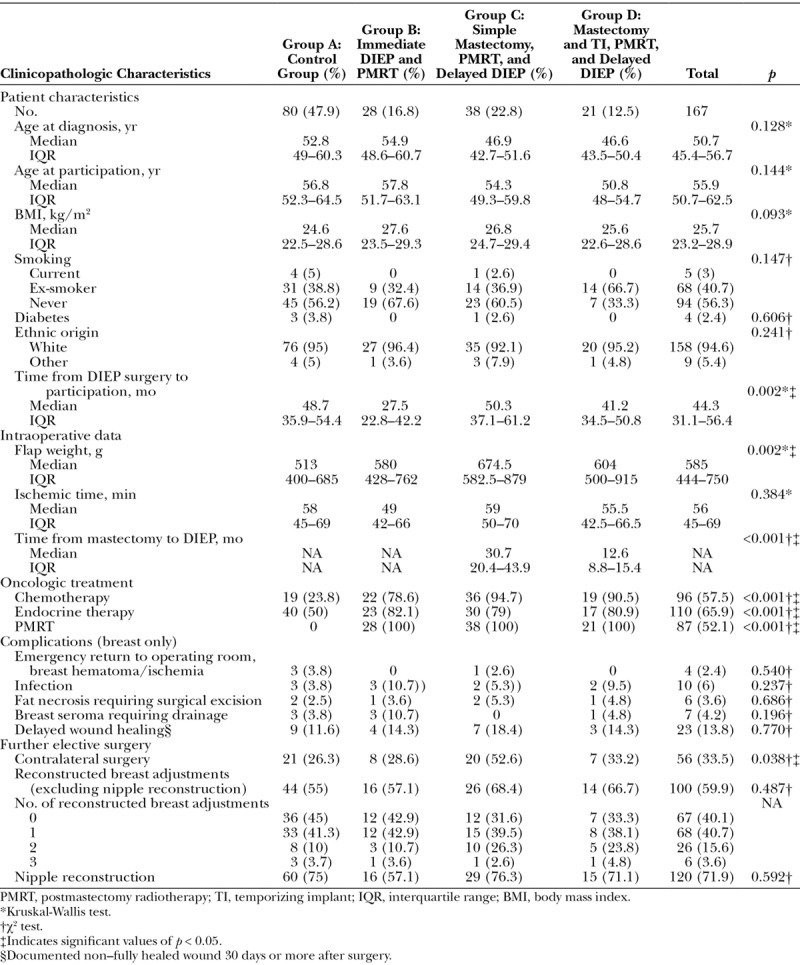
Table 1 demonstrates the clinicopathologic features of each group according to radiotherapy and timing of surgery. The patient characteristics were broadly similar in the four groups. There was a significant difference between the groups when comparing time from surgery to participation in the study; it was shortest for group B (immediate reconstruction with postmastectomy radiotherapy). Recently, more patients have undergone immediate reconstruction and then radiotherapy, as the team is offering immediate reconstruction to selected women who require adjuvant radiotherapy, and simultaneously the indications for radiotherapy have become broader. As expected, participants in group A (no postmastectomy radiotherapy) had significantly lower rates of chemotherapy and endocrine therapy because they had ductal carcinoma in situ or less advanced invasive disease.
Table 1.
Clinicopathologic Data According to the Four Participant Groups and the Total
Panel Assessment
The weighted kappa agreement between the four assessors in the panel assessment was “moderate” (0.4) for the global score. For the subcategories, the weighted kappa agreements were “slight” for nipple-areola appearance (0.2) and scarring (0.29), and “fair” for volume (0.31), shape (0.36), and symmetry (0.38). Because of the low agreement in the subcategories, only the global score was used in further analysis. Figure 3 demonstrates an image of a typical patient from each patient pathway.
Fig. 3.
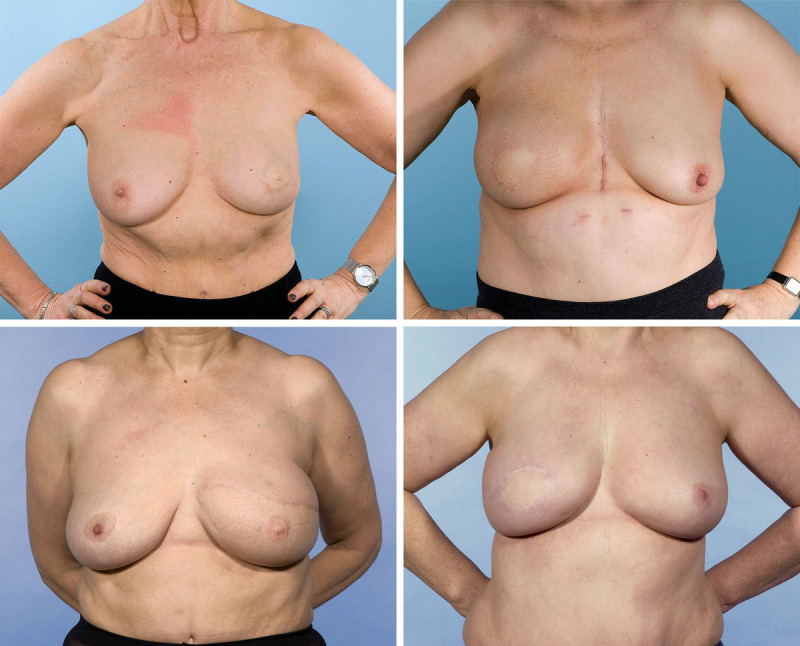
Representative images from each of the four groups. Please note that the panel assessed moving three-dimensional images not the two-dimensional images shown here. (Above, left) Patient with a score of 8.5 from group A: skin-sparing mastectomy with immediate DIEP flap reconstruction without postmastectomy radiotherapy (median score, 8). (Above, right) Patient with a score of 7.25 from group B: skin-sparing mastectomy with immediate DIEP flap reconstruction and postmastectomy radiotherapy (median score, 7.3). (Below, left) Patient with a score of 7.0 from group C: simple mastectomy (no skin preservation), postmastectomy radiotherapy, and delayed DIEP flap reconstruction (median score, 7.3). (Below, right) Patient with a score of 7.75 from group D: skin-sparing mastectomy and preservation of skin flaps with temporizing implant, postmastectomy radiotherapy, and later DIEP reconstruction (median score, 7.8).
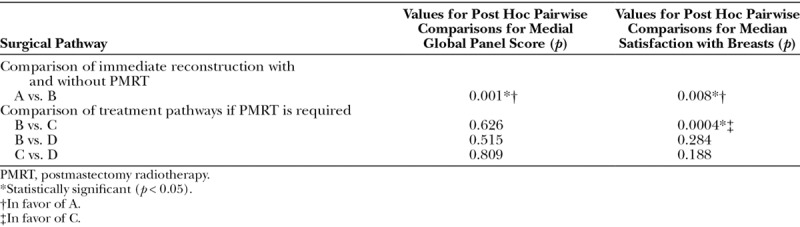
The median global panel scores were significantly different when the patients were divided according to the treatment pathway (p = 0.001, Kruskal-Wallis test) (Table 2 and Fig. 4). Post hoc pairwise comparisons demonstrated that the panel gave a significantly worse score to an irradiated immediate DIEP flap reconstruction (group B) than to an unirradiated DIEP flap reconstruction (group A controls). However, there were no differences in median panel scores among groups B, C, and D, all of whom received postmastectomy radiotherapy (Table 3).
Table 2.
Median Global Panel Scores and Satisfaction with Breasts According to Surgical Pathway
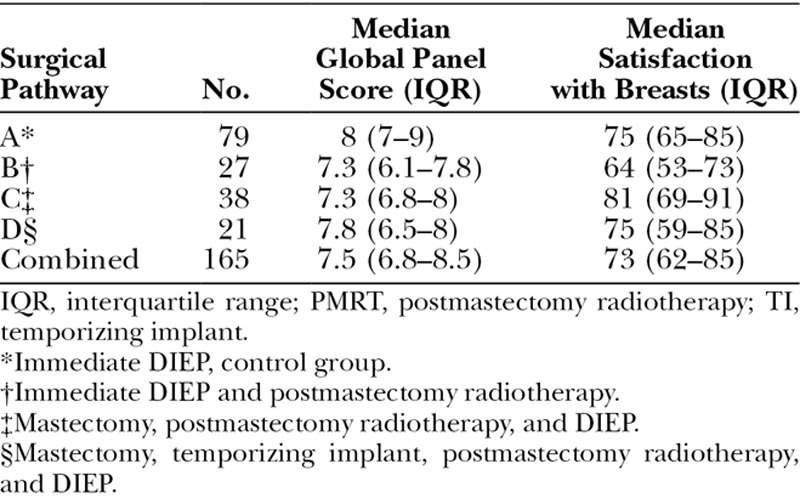
Fig. 4.
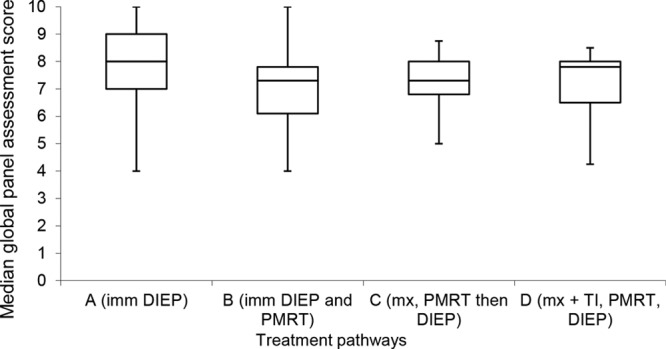
Box-and-whisker plot demonstrating global panel assessment scores according to different treatment pathways. The horizontal line through the center of each box represents the median score, outer horizontal lines of each box represents upper and lower quartiles, and the ends of the vertical lines represent minimum and maximum scores. There was a significant difference between A and B. imm, immediate; PMRT, postmastectomy radiotherapy; mx, mastectomy; TI, temporizing implant.
Table 3.
Pairwise Comparisons of Median Global Panel Scores and Satisfaction with Breasts According to Surgical Pathway (p Values)
Patient Satisfaction
The median satisfaction with breasts scores were significantly different between some groups (p = 0.002, Kruskal-Wallis test) (Table 2 and Fig. 5). Post hoc pairwise comparison again demonstrated a significant difference according to whether postmastectomy radiotherapy was administered to an immediate DIEP flap reconstruction. Among women who had undergone radiotherapy, those who had an irradiated immediate reconstruction (group B) had significantly lower satisfaction than those who underwent delayed DIEP flap surgery after simple mastectomy (group C). There was no significant difference between immediate reconstruction with radiotherapy (group B) and mastectomy with temporizing implant, radiotherapy, and then DIEP flap surgery (group D) (Table 3).
Fig. 5.
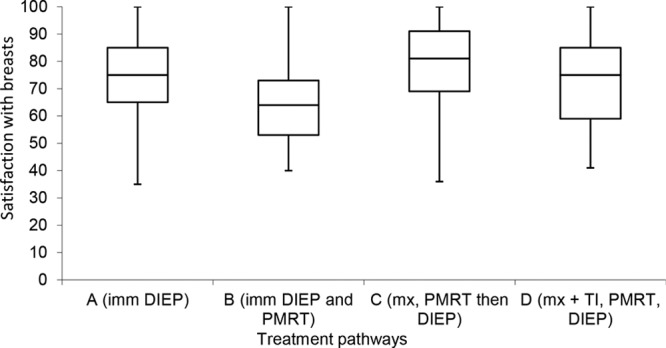
Box-and-whisker plot demonstrating Satisfaction with Breasts according to different treatment pathways. The horizontal line through the center of each box represents median score, outer horizontal lines of each box represent upper and lower quartiles, and the ends of the vertical lines represent minimum and maximum scores. There was a significant difference between A and B, and B and C. imm, immediate; PMRT, postmastectomy radiotherapy; mx, mastectomy; TI, temporizing implant.
DISCUSSION
The timing of autologous reconstruction in the context of a predicted need for postmastectomy radiotherapy poses a challenge to breast and plastic surgeons. This study has demonstrated that irradiating an immediate DIEP flap reconstruction does have a detrimental effect on aesthetic outcome as judged by a panel of surgeons and by patients themselves in their response to the Satisfaction with Breasts domain of the BREAST-Q questionnaire.
However, for those women who required postmastectomy radiotherapy, there was no significant difference in panel assessment of aesthetic outcome between immediate DIEP flap reconstruction with radiotherapy, simple mastectomy with radiotherapy then delayed DIEP flap surgery, or mastectomy and temporizing implant with radiotherapy and then conversion to DIEP flap surgery. Patient-reported outcomes did show differences, with women undergoing delayed reconstruction after a simple mastectomy the most satisfied, but there was no significant difference between women with an irradiated immediate DIEP flap reconstruction and those who had undergone mastectomy with temporizing implant, postmastectomy radiotherapy, and later DIEP flap surgery. It is known that women who have a delayed reconstruction report high levels of satisfaction because they have been living with a flat chest wall, whereas women who undergo immediate reconstruction are comparing their reconstruction with their natural breast.30 Furthermore, many women who received radiotherapy to their DIEP flap were not expected to require adjuvant radiotherapy. This disappointment may be reflected in the satisfaction scores whether or not there was a substantial change in the reconstruction after radiotherapy. These results demonstrate one of the potential pitfalls of using patient-reported outcomes to compare patients who have taken a different treatment pathway to reach the same endpoint.
Women who are offered a temporizing implant are often those who would ideally like to undergo immediate autologous reconstruction but have been advised to delay the use of their autologous flap until after radiotherapy, and thus are the most relevant comparator group to the irradiated DIEP flap cohort. The finding that there is no significant difference between these two groups in terms of patient satisfaction or panel assessment suggests that these are both reasonable treatment pathways as far as patient satisfaction and cosmetic outcome are concerned.
There is a paucity of data on the aesthetic impact of irradiating autologous reconstructions in the literature. Early studies of transverse rectus abdominis myocutaneous and DIEP flaps concluded that radiotherapy had resulted in an increase in late complications and requirement for reoperation, and had a negative impact on aesthetic appearance, symmetry, contracture, and hyperpigmentation of the skin.31–34 Similarly, initial studies of patient-reported aesthetic outcome lacked a comparator group35,36 or included small numbers of women and a wide variety of breast reconstruction techniques.37 Although these early studies involved relatively few patients, they established the mindset that irradiating an autologous abdominal flap gives unacceptable results and that delayed reconstruction is preferable.
However, plastic surgical techniques continue to improve, and DIEP flaps have become routine, largely replacing transverse rectus abdominis myocutaneous flaps. Careful planning38 and maneuvers designed to improve flap design and circulation are all likely to protect against radiotherapy toxicity. Simultaneously, advances in postmastectomy radiotherapy planning, fractionation, and delivery directed at reducing dose heterogeneity and damage to normal tissues contribute to a lower impact on autologous flaps than has previously been reported. The literature is beginning to reflect this, with a meta-analysis of outcome and a systematic review of patient satisfaction both reporting acceptable outcomes.39,40 Clarke-Pearson et al.41 carried out the most direct comparison, reporting on postoperative photographs of 11 cases of bilateral DIEP reconstruction with unilateral postmastectomy radiotherapy. They found good aesthetic outcome with satisfactory preservation of breast shape and minimal change in flap consistency on manual compression (by the patient in the photograph). A recent study from China demonstrated that radiation and its timing did not have an adverse impact on patients’ aesthetic and psychological evaluations by the BREAST-Q questionnaire. In fact, they found that there was no significant difference in satisfaction whether the patient underwent immediate reconstruction with radiotherapy or a delayed reconstruction after radiotherapy.42
The advantage of our study was that it is relatively large and the pairwise comparisons were made to answer clinical questions. This allowed separate comparison of the impact of postmastectomy radiotherapy on an immediate DIEP flap reconstruction (A versus B) and of the final outcomes in women who underwent radiotherapy with a variety of reconstructive surgery pathways (B versus C versus D). The latter will aid decision-making for women requiring radiotherapy as to whether to choose an immediate or delayed reconstruction with or without a temporizing implant.
Although the number of participants in the subgroups was small and the study was retrospective, it gives weight to the argument that autologous reconstruction can be considered even when postmastectomy radiotherapy is anticipated. It was disappointing that the agreement among the panel raters was low for the subcomponents of the aesthetic outcome and therefore was not used in the analysis of the results. After careful consideration with statisticians, it was decided that the moderate agreement of the global score was sufficient to take forward to the analysis. We believe that having the opinion of the surgeons in addition to the patient-reported outcome measures is important to help provide an unbiased opinion of the reconstruction. The psychological impact of undergoing cancer treatment, premorbid psychological state, and treatment pathway factors (i.e., going from flat chest to reconstruction versus going from native breast to reconstruction) to which the patients are exposed are likely to impact on their satisfaction.
If the option of immediate reconstruction is offered to more women who are expected to have postmastectomy radiotherapy, the surgeon is able to counsel women about the likely changes and manage their expectations, and can take measures such as making the reconstruction larger to mitigate against volume loss from radiation-induced fibrosis. Thus, if this becomes routine, patient satisfaction with the outcome may be better than in this study.
CONCLUSIONS
This study has demonstrated that postmastectomy radiotherapy to an immediate DIEP flap reconstruction does have a detrimental effect on aesthetic outcome as judged by a panel of surgeons and by patients themselves. There was no significant difference in panel assessment among the three treatment pathways in women who required postmastectomy radiotherapy, whereas women undergoing delayed reconstruction reported greatest satisfaction. The lack of a difference in patient satisfaction and global panel assessment between the irradiated immediate DIEP flap reconstruction and the mastectomy with temporizing implant groups suggests that a careful and individualized discussion must take place such that women are able choose between the two options, both of which have benefits and disadvantages, according to their own priorities and the surgeon’s judgment on suitability.
ACKNOWLEDGMENTS
Rachel L. O’Connell, M.R.C.S., was funded by a 1-year Royal College of Surgeons of England Research Fellowship. The Royal Marsden/Institute for Cancer Research is a National Institute of Health Research Biomedical Research Centre, and this support is acknowledged. The authors acknowledge the assistance of Jon Knox, advanced nurse practitioner, plastic surgery, and Giuseppe Catanuto, M.D., consultant oncoplastic surgeon, Azienda Ospedaliera Cannizzaro, Catania, Italy.
The BREAST-Q is copyrighted by Memorial Sloan-Kettering Cancer Center and The University of British Columbia © 2006, all rights reserved. For BREAST-Q information and permission to use, contact MAPI Research Trust, Lyon, France; e-mail: PROinformation@mapi-trust.org. Available at: www.mapi-trust.org.
Footnotes
This trial is registered under the name “Impact of Radiotherapy on Patients Undergoing DIEP Flap Breast Reconstruction,” clinicaltrials.gov identification number NCT03072316 (https://clinicaltrials.gov/ct2/show/NCT03072316).
Disclosure: The authors have no commercial associations or financial disclosures.
REFERENCES
- 1.Second All Breast Cancer Report. Available at: http://www.ncin.org.uk/cancer_type_and_topic_specific_work/cancer_type_specific_work/breast_cancer/. Accessed June 30, 2018.
- 2.Jeevan R, Cromwell D, Browne J, et al. National mastectomy and breast reconstruction audit. Available at: https://associationofbreastsurgery.org.uk/media/1083/nmbra-annual-report-2009.pdf. Accessed June 30, 2018.
- 3.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150:9. [DOI] [PubMed] [Google Scholar]
- 4.Recht A, Edge SB. Evidence-based indications for postmastectomy irradiation. Surg Clin North Am. 2003;83:995. [DOI] [PubMed] [Google Scholar]
- 5.Dragun AE, Huang B, Gupta S, Crew JB, Tucker TC. One decade later: Trends and disparities in the application of post-mastectomy radiotherapy since the release of the American Society of Clinical Oncology clinical practice guidelines. Int J Radiat Oncol Biol Phys. 2012;83:e591. [DOI] [PubMed] [Google Scholar]
- 6.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116. [DOI] [PubMed] [Google Scholar]
- 7.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949. [DOI] [PubMed] [Google Scholar]
- 8.Clarke M, Collins R, Darby S, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;366:2087. [DOI] [PubMed] [Google Scholar]
- 9.McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truong PT, Olivotto IA, Kader HA, Panades M, Speers CH, Berthelet E. Selecting breast cancer patients with T1-T2 tumors and one to three positive axillary nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1337. [DOI] [PubMed] [Google Scholar]
- 11.Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg. 1994;32:32. [DOI] [PubMed] [Google Scholar]
- 12.Kelley BP, Ahmed R, Kidwell KM, Kozlow JH, Chung KC, Momoh AO. A systematic review of morbidity associated with autologous breast reconstruction before and after exposure to radiotherapy: Are current practices ideal? Ann Surg Oncol. 2014;21:1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: A critical review of the literature. Plast Reconstr Surg. 2009;124:395. [DOI] [PubMed] [Google Scholar]
- 14.Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg. 2004;113:1617. [DOI] [PubMed] [Google Scholar]
- 15.Kronowitz SJ. Delayed-immediate breast reconstruction: Technical and timing considerations. Plast Reconstr Surg. 2010;125:463. [DOI] [PubMed] [Google Scholar]
- 16.Cohen WA, Mundy LR, Ballard TN, et al. The BREAST-Q in surgical research: A review of the literature 2009-2015. J Plast Reconstr Aesthet Surg. 2016;69:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pusic AL, Chen CM, Cano S, et al. Measuring quality of life in cosmetic and reconstructive breast surgery: A systematic review of patient-reported outcomes instruments. Plast Reconstr Surg. 2007;120:823. [DOI] [PubMed] [Google Scholar]
- 18.Pusic AL. Measuring quality of life in breast surgery: Content development of a new modular system to capture patient-reported outcomes (The MSKCC Breast-Q). Paper presented at: 13th Annual Conference of the International Society of Quality of Life Research; October 10–14, 2006; Lisbon, Portugal. [Google Scholar]
- 19.Visser NJ, Damen TH, Timman R, Hofer SO, Mureau MA. Surgical results, aesthetic outcome, and patient satisfaction after microsurgical autologous breast reconstruction following failed implant reconstruction. Plast Reconstr Surg. 2010;126:26. [DOI] [PubMed] [Google Scholar]
- 20.Maass SW, Bagher S, Hofer SO, Baxter NN, Zhong T. Systematic review: Aesthetic assessment of breast reconstruction outcomes by healthcare professionals. Ann Surg Oncol. 2015;22:4305. [DOI] [PubMed] [Google Scholar]
- 21.Veiga DF, Neto MS, Garcia EB, et al. Evaluations of the aesthetic results and patient satisfaction with the late pedicled TRAM flap breast reconstruction. Ann Plast Surg. 2002;48:515. [DOI] [PubMed] [Google Scholar]
- 22.Haekens CM, Enajat M, Keymeulen K, Van der Hulst RR. Self-esteem and patients’ satisfaction after deep inferior epigastric perforator flap breast reconstruction. Plast Surg Nurs. 2011;31:160. [DOI] [PubMed] [Google Scholar]
- 23.Ramon Y, Ullmann Y, Moscona R, et al. Aesthetic results and patient satisfaction with immediate breast reconstruction using tissue expansion: A follow-up study. Plast Reconstr Surg. 1997;99:686. [DOI] [PubMed] [Google Scholar]
- 24.Behranwala KA, Dua RS, Ross GM, Ward A, A’hern R, Gui GP. The influence of radiotherapy on capsule formation and aesthetic outcome after immediate breast reconstruction using biodimensional anatomical expander implants. J Plast Reconstr Aesthet Surg. 2006;59:1043. [DOI] [PubMed] [Google Scholar]
- 25.Rietjens M, De Lorenzi F, Venturino M, Petit JY. The suspension technique to avoid the use of tissue expanders in breast reconstruction. Ann Plast Surg. 2005;54:467. [DOI] [PubMed] [Google Scholar]
- 26.Castelló JR, Garro L, Nájera A, Mirelis E, Sánchez-Olaso A, Barros J. Immediate breast reconstruction in two stages using anatomical tissue expansion. Scand J Plast Reconstr Surg Hand Surg. 2000;34:167. [DOI] [PubMed] [Google Scholar]
- 27.Cocquyt VF, Blondeel PN, Depypere HT, et al. Better cosmetic results and comparable quality of life after skin-sparing mastectomy and immediate autologous breast reconstruction compared to breast conservative treatment. Br J Plast Surg. 2003;56:462. [DOI] [PubMed] [Google Scholar]
- 28.Hayes AJ, Garner JP, Nicholas W, Laidlaw IJ. A comparative study of envelope mastectomy and immediate reconstruction (EMIR) with standard latissimus dorsi immediate breast reconstruction. Eur J Surg Oncol. 2004;30:744. [DOI] [PubMed] [Google Scholar]
- 29.Hernanz F, Regaño S, Redondo-Figuero C, Orallo V, Erasun F, Gómez-Fleitas M. Oncoplastic breast-conserving surgery: Analysis of quadrantectomy and immediate reconstruction with latissimus dorsi flap. World J Surg. 2007;31:1934. [DOI] [PubMed] [Google Scholar]
- 30.National Mastectomy and Breast Reconstruction Audit. Fourth Annual Report 2011. Available at: https://www.rcseng.ac.uk/standards-and-research/research/clinical-effectiveness-unit/documents-and-publications/. Accessed June 30, 2018.
- 31.Tran NV, Chang DW, Gupta A, Kroll SS, Robb GL. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2001;108:78. [DOI] [PubMed] [Google Scholar]
- 32.Spear SL, Ducic I, Low M, Cuoco F. The effect of radiation on pedicled TRAM flap breast reconstruction: Outcomes and implications. Plast Reconstr Surg. 2005;115:84. [PubMed] [Google Scholar]
- 33.Leonardi MC, Garusi C, Santoro L, et al. Impact of medical discipline and observer gender on cosmetic outcome evaluation in breast reconstruction using transverse rectus abdominis myocutaneous (TRAM) flap and radiotherapy. J Plast Reconstr Aesthet Surg. 2010;63:2091. [DOI] [PubMed] [Google Scholar]
- 34.Rogers NE, Allen RJ. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plast Reconstr Surg. 2002;109:1919. [DOI] [PubMed] [Google Scholar]
- 35.Huang CJ, Hou MF, Lin SD, et al. Comparison of local recurrence and distant metastases between breast cancer patients after postmastectomy radiotherapy with and without immediate TRAM flap reconstruction. Plast Reconstr Surg. 2006;118:1079. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman RP, Mark RJ, Kim AI, et al. Radiation tolerance of transverse rectus abdominis myocutaneous-free flaps used in immediate breast reconstruction. Am J Clin Oncol. 1998;21:381. [DOI] [PubMed] [Google Scholar]
- 37.Adesiyun TA, Lee BT, Yueh JH, et al. Impact of sequencing of postmastectomy radiotherapy and breast reconstruction on timing and rate of complications and patient satisfaction. Int J Radiat Oncol Biol Phys. 2011;80:392. [DOI] [PubMed] [Google Scholar]
- 38.Ghattaura A, Henton J, Jallali N, et al. One hundred cases of abdominal-based free flaps in breast reconstruction: The impact of preoperative computed tomographic angiography. J Plast Reconstr Aesthet Surg. 2010;63:1597. [DOI] [PubMed] [Google Scholar]
- 39.Schaverien MV, Macmillan RD, McCulley SJ. Is immediate autologous breast reconstruction with postoperative radiotherapy good practice? A systematic review of the literature. J Plast Reconstr Aesthet Surg. 2013;66:1637. [DOI] [PubMed] [Google Scholar]
- 40.El-Sabawi B, Ho AL, Sosin M, Patel KM. Patient-centered outcomes of breast reconstruction in the setting of post-mastectomy radiotherapy: A comprehensive review of the literature. J Plast Reconstr Aesthet Surg. 2017;70:768. [DOI] [PubMed] [Google Scholar]
- 41.Clarke-Pearson EM, Chadha M, Dayan E, et al. Comparison of irradiated versus nonirradiated DIEP flaps in patients undergoing immediate bilateral DIEP reconstruction with unilateral postmastectomy radiation therapy (PMRT). Ann Plast Surg. 2013;71:250. [DOI] [PubMed] [Google Scholar]
- 42.He S, Yin J, Robb GL, et al. Considering the optimal timing of breast reconstruction with abdominal flaps with adjuvant irradiation in 370 consecutive pedicled transverse rectus abdominis myocutaneous flap and free deep inferior epigastric perforator flap performed in a Chinese oncology center: Is there a significant difference between immediate and delayed? Ann Plast Surg. 2017;78:633. [DOI] [PMC free article] [PubMed] [Google Scholar]


