Abstract
Gestational diabetes mellitus (GDM) is associated with adverse perinatal outcomes. This study aimed to examine the association between GDM and abnormal vaginal flora, and the association between abnormal vaginal flora and adverse pregnancy outcomes.
This was a prospective study of pregnant women who visited Xuanwu Hospital of the Capital Medical University (Beijing, China) between February and October 2015. All women were screened for GDM according to the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommendations. Vaginal secretions were sampled at 28 to 30 and 37 to 40 weeks. Microorganisms were examined.
The women were 28.3 ± 2.6 years and their body mass index was 22.8 ± 1.4 kg/m2. GDM was associated with higher frequencies of vulvovaginal candidiasis (22.6% vs 9.7%, P < .001), premature rupture of membranes (PROM) (22.6% vs 11.5%, P = .004), premature delivery (16.1% vs 5.5%, P = .02), chorioamnionitis/puerperal infection (19.4% vs 4.5%, P < .001), macrosomia (9.7% vs 4.0%, P = .04), neonatal hypoglycemia (5.4% vs 1.0%, P = .02), and neonatal referral (15.1% vs 6.5%, P = .008). Among healthy women, abnormal flora was associated with PROM (19.4% vs 7.5%, P = .02) and chorioamnionitis/puerperal infection (11.9% vs 0.8%, P < .001). Among women with GDM, abnormal flora was associated with PROM (32.1% vs 10.0%, P < .001), premature delivery (17.7% vs 6.3%, P = .04), and chorioamnionitis/puerperal infection (32.8% vs 2.5%, P < .001).The vaginal infection rate was higher in patients with GDM compared with healthy pregnant women. GDM and abnormal vaginal flora were both associated with adverse pregnancy outcomes. The vaginal Lactobacillus species were different between the 2 groups, which could contribute to the adverse outcomes.
Keywords: bacterial vaginosis, gestational diabetes mellitus, lactobacillus, perinatal outcomes, vaginal microbiology, vulvovaginal candidiasis
1. Introduction
Gestational diabetes mellitus (GDM) is a type of diabetes diagnosed during the 2nd or 3rd trimester of pregnancy and that is clearly not overt diabetes.[1] Women with diabetes during the 1st trimester are classified as having preexisting pregestational diabetes.[1] Recently, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) issued new criteria for GDM screening and diagnosis.[2,3] According to the IADPSG recommendations, fasting plasma glucose should be measured at the first prenatal visit and women with fasting blood glucose (FBG) >7.0 mmol/L will be diagnosed with overt DM and those with FBG >5.1 mmol/L will be diagnosed with GDM. For women with FBG < 5.1 mmol/L, a 75-g 2-hour oral glucose tolerance test (OGTT) should be performed at 24 to 28 weeks: those with FBG >7.0 mmol/L will be diagnosed with overt DM; those with FBG >5.1 mmol/L, 1-hour glucose >10 mmol/L, and 2-hour glucose >8.5 mmol/L will be diagnosed with GDM; and all others will be considered insulin sensitive.[2,3] According to the IADPSG criteria, Zhu et al[4] conducted a retrospective study of 17,186 pregnant women from 13 hospitals in China between 2010 and 2011, and the incidence of GDM was found to be 17.5%. In the Arab Emirates, Agarwal et al[2] showed that the use of the IADPSG criteria increased the prevalence of GDM nearly 3-fold (from 12.9% using the conventional criteria, to 37.7% when using IADPSG), but the IADPSG criteria significantly simplified the diagnosis of GDM by circumventing a large number of OGTTs. In Caucasians populations, the overall prevalence of GDM was found to be 17.8% (range, 9.3%–25.5%).[5,6]
GDM is associated with increased risk of maternal complications such as spontaneous preterm birth, premature rupture of membrane (PROM), chorioamnionitis, traumatic complications of vaginal delivery, cesarean delivery, morbidity from operative delivery, preeclampsia, and risk of developing metabolic syndrome or DM.[7–11] GDM is also associated with significant risk for the fetus, including stillbirth, macrosomia, shoulder dystocia, and congenital malformations.[11–15] Neonatal complications are also more frequent and include neonatal hypoglycemia, neonatal hyperbilirubinemia, hypocalcemia, erythrocytosis, and poor feeding.[7,11,16]
The vaginal flora encompasses the microorganisms that colonize the vagina. The primary colonizing bacteria in healthy women are Lactobacillus and the lactic acid, and they produce protection against pathogenic species such as Candida.[17] The vaginal flora is highly sensitive to disturbances such as menses, sexual intercourse, vaginal douching, and contraception.[17] In addition, pregnancy is associated with an immunocompromised state that increases the susceptibility to vaginal Candida infection.[18] In itself, vaginal infection can be a cause of intrauterine infections, stillbirth, premature delivery, and neurological damage to the fetus.[19–22] Since GDM is associated with poor metabolic control, higher body mass index, and impaired leukocyte function,[23,24] some studies have suggested that GDM is associated with disturbances in the vaginal flora and vagina infections,[25–30] but this is controversial.[31] Infections caused by Candida are relatively well known to be associated with GDM,[25–28] but much less is known about bacterial infections.
Based on those facts, we hypothesized that abnormal vaginal flora (such as changes in dominant bacterial strains and pathogenic bacterial infection) in patients with GDM is associated with adverse perinatal outcomes. Therefore, the aim of this study was to examine the association between GDM and abnormal vaginal flora, and the association between abnormal vaginal flora and adverse maternal and neonatal outcomes. In addition, high-throughput sequencing was used to reveal the composition and dynamic changes of the vaginal flora in patients with GDM during pregnancy. The results of this study could provide some clues about the pathogenesis of GDM, associated conditions, and potential targets for prophylaxis and treatment.
2. Methods
2.1. Study design and patients
This was a prospective study of pregnant women who visited the Xuanwu Hospital of Capital Medical University (Beijing, China) between February and October 2015. They underwent an OGTT at 24 to 28 weeks of pregnancy. The study was approved by the ethics committee of the Xuanwu Hospital of Capital Medical University (Beijing, China). All patients provided written informed consent.
All women were screened and diagnosed for GDM according to the IADPSG recommendations.[2,3] The patients were grouped according to with or without GDM (controls). The inclusion criteria were Han nationality; singleton pregnancy; without sex in the past week; not taking systemic or local antibiotic; and without medical history of vaginal douching or treatment.
2.2. Sampling and data collection
Vaginal secretions were collected from the upper third of the vagina with sterile vaginal swabs, avoiding contact with the orificium vaginae or the vulva to prevent contamination. In both groups, the samples were collected at 28 to 30 and 37 to 40 weeks of pregnancy. Vaginal pH was measured and the distribution of the microorganisms was observed and assessed[32]: vaginal microecology with a roughly normal pattern: (grade II–III vaginal flora density; grade II–III vaginal flora diversity; Lactobacillus is the dominant bacteria; 0–5 pyocytes or leukocytes per high-magnification fields); bacterial vaginosis (BV): according to the criteria proposed by Nugent et al,[33] BV was diagnosed upon a total score ≥7; vulvovaginal candidiasis (VVC): VVC was diagnosed when spores and pseudohyphae were detected under the microscope; and BV plus VVC (BVC): co-occurrence of BV and VVC. The samples were stored at −80 °C for sequencing.
2.3. Sequencing and bioinformatics analysis
In the GDM group, 30 samples were collected from 15 GDM patients. In the healthy control group, 20 samples were collected from 10 healthy pregnant women. Total DNA was extracted from the vaginal secretions. Amplicon libraries of the V4-V5 hyper-variable regions of the 16S rRNA gene were generated using specific primers. Target amplicon fragments were recovered with magnetic beads to build the library. Sequencing was conducted using a MiSeg high-throughput sequencing platform (Illumina, Inc., San Diego, CA).
Raw sequencing data were processed by removing low-quality reads to acquire clean data.[34] Then, the Fast Length Adjustment of Short reads, v1.2.11 (FLASH) software was used to assemble paired-end reads and to screen under specific standards.[35] Finally, clean tags of the V4-V5 hyper-variable regions were sorted out. The USEARCH (v7.0.1090) software was applied to cluster the clean tags into operational taxonomic units (OTU).[36] Then the nonphylogenetic alpha diversity, phylogenetic beta diversity, and taxonomic composition analyses were performed on the OTU table using the open-source bioinformatics pipeline QIIME.[37]
2.4. Follow-up
Telephone follow-up was conducted about perinatal outcomes of maternal and neonatal complications in both groups.
2.5. Statistical analysis
SPSS 13.0 (SPSS Inc, Chicago, IL) was used for statistical analysis. Continuous data were expressed as means ± standard deviations and analyzed using the Student t test. Categorical data were expressed as frequencies and analyzed using the chi-square test. Two-sided P-values < .05 were considered statistically significant.
3. Results
3.1. Characteristics of the patients
A total of 386 pregnant women were enrolled, including 186 with GDM and 200 healthy controls. The patients were 28.3 ± 2.6 years and their body mass index (BMI) was 22.8 ± 1.4 kg/m2 (range, 18–28 kg/m2).
3.2. GDM is associated with abnormal vaginal flora
In the healthy control group, vaginal pH was 3.72 ± 0.20; VVC frequency was 9.7% (18/200); BV frequency was 9.5% (19/200); BVC frequency was 5.0% (10/200); the frequency of normal bacteria suppression was 1.5% (3/200); and the frequency of changes in dominant bacteria strains was 8.5% (17/200). In the GDM group, vaginal pH was 3.46 ± 0.23; VVC frequency was 22.6% (42/186); BV frequency was 10.4% (19/186); BVC frequency was 6.0% (12/186); the frequency of normal bacteria suppression was 2.7% (5/186); and the frequency of changes in dominant bacteria strains was 15.1% (28/186). The GDM group showed a significantly higher frequency of VVC (22.6% vs 9.7%, P < .001) and a lower frequency of normal vaginal flora (43.0% vs 66.5%, P < .001) (Table 1).
Table 1.
Comparisons of the vaginal flora.
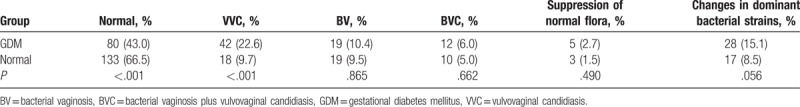
3.3. GDM is associated with worst maternal outcomes
The frequencies of PROM, premature delivery, and chorioamnionitis (confirmed by postoperative placental pathology) or puerperal infection were higher in the GDM group compared with the control group (22.6% vs 11.5%, P = .004; 16.1% vs 5.5%, P = .02; 19.4% vs 4.5%, P < .001, respectively) (Table 2).
Table 2.
Comparisons of the maternal outcomes in the perinatal stage.
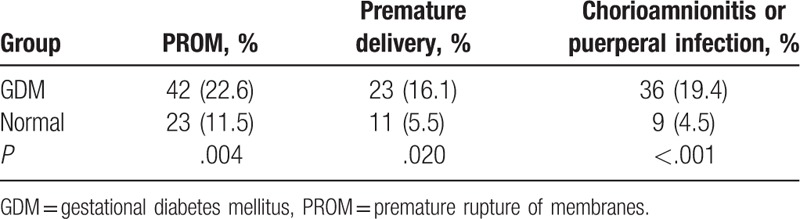
3.4. Association of GDM with worst neonatal outcomes
Compared with the healthy control group, the GDM group showed higher frequencies of macrosomia (9.7% vs 4.0%, P = .04), neonatal hypoglycemia (5.4% vs 1.0%, P = .02), and neonatal referral rate (15.1% vs 6.5%, P = .008) (Table 3). There were no differences between the 2 groups regarding fetal growth restriction and mild asphyxia (both P > .05).
Table 3.
Comparisons of the neonatal outcomes in the perinatal stage.
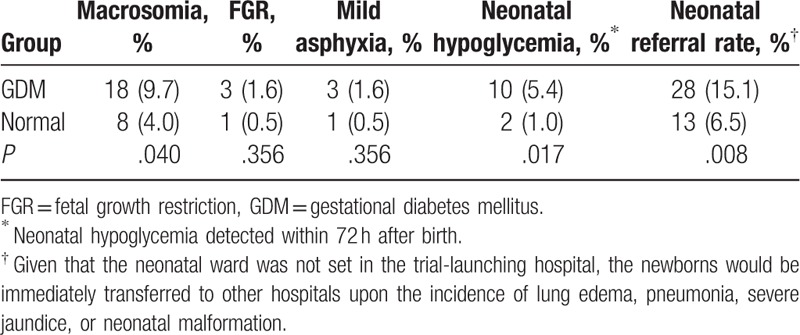
3.5. Abnormal vaginal flora is associated with adverse perinatal outcomes among healthy women
Among the 200 healthy pregnant women, 67 had an abnormal vaginal flora. Compared with the women with a normal flora, those with an abnormal flora showed higher frequencies of PROM (19.4% vs 7.5%, P = .02) and chorioamnionitis or puerperal infection (11.9% vs 0.8%, P < .001), while there was no difference regarding premature delivery (P = .19) (Table 4).
Table 4.
Association between the vaginal flora and perinatal outcomes in healthy pregnant women.
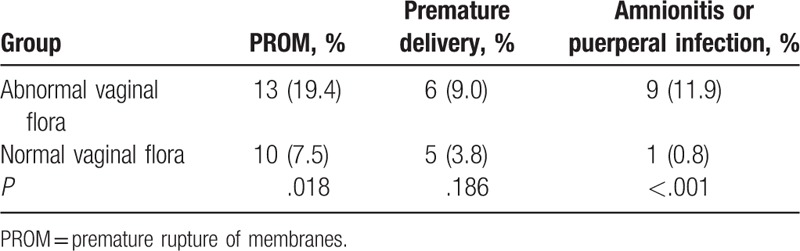
3.6. Abnormal vaginal flora is associated with adverse perinatal outcomes among patients with GDM
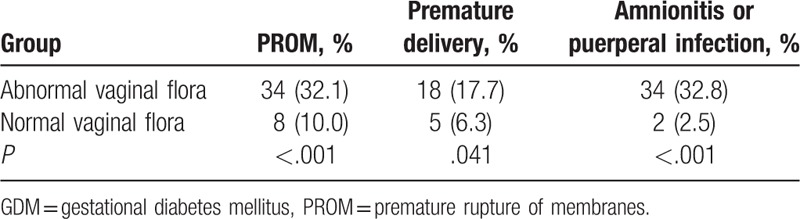
Among the 186 pregnant women with GDM, 106 carried an abnormal vaginal flora. Compared with the patients with GDM and a normal flora, those with an abnormal flora showed higher frequencies of PROM (32.1% vs 10.0%, P < .001), premature delivery (17.7% vs 6.3%, P = .04), and chorioamnionitis or puerperal infection (32.8% vs 2.5%, P < .001) (Table 5).
Table 5.
Association between the vaginal flora and perinatal outcomes in pregnant women with GDM.
3.7. Changes in the composition of the vaginal flora during pregnancy
Among the 30 specimens from the GDM group and the 20 specimens from the control group and after primer removal, 1,909,133 clean tags were obtained from all samples (average of 38,182 ± 454 tags/sample and average length of 378 ± 1 base pairs). Clean tags were clustered into OTUs based on 97% similarity: 169 OTUs arose from the 50 samples. By blasting with databases, the taxonomy of OTUs was determined at the levels of phylum (Fig. 1), genus (Fig. 2), and species (Fig. 3).
Figure 1.
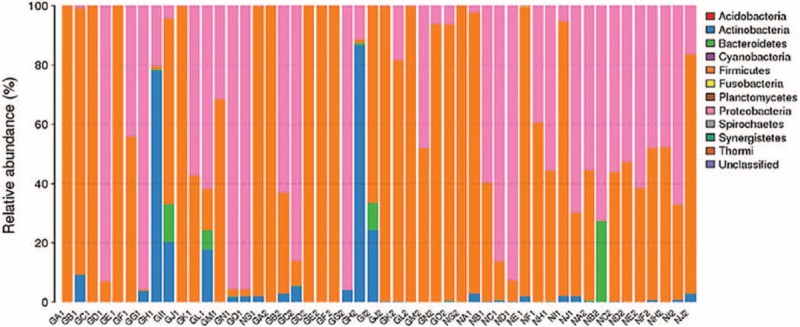
The taxonomic composition of the vaginal flora from the gestational diabetes mellitus (GDM) group (GA-GO) and the control group (NA-NJ) at the phylum level and based on 16S rRNA gene sequencing. For both groups, the samples of vaginal secretions collected at 28 to 30 weeks of pregnancy were numbered as “1” (e.g., GA1, NA1, ...), while those 37 to 40 weeks of pregnancy were accordingly as “2” (e.g. GA2, NA2, ...).
Figure 2.
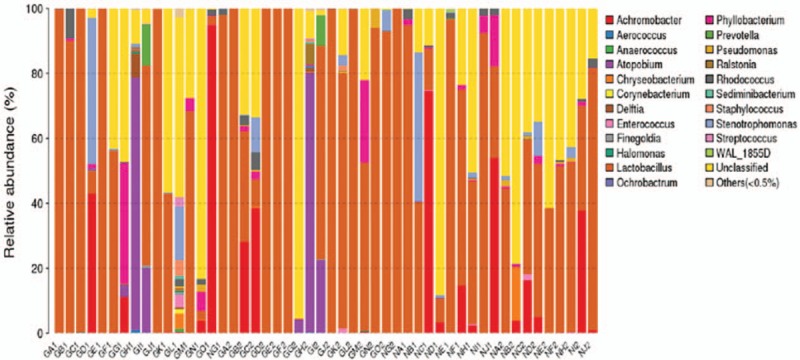
The taxonomic composition of the vaginal flora from the gestational diabetes mellitus (GDM) group (GA-GO) and the control group (NA-NJ) at the genus level and based on 16S rRNA gene sequencing. For both groups, the samples of vaginal secretion collected at 28 to 30 weeks of pregnancy were numbered as “1” (e.g., GA1, NA1, ...), while those from 37 to 40 weeks of pregnancy were numbered as “2” (e.g., GA2, NA2, ...).
Figure 3.
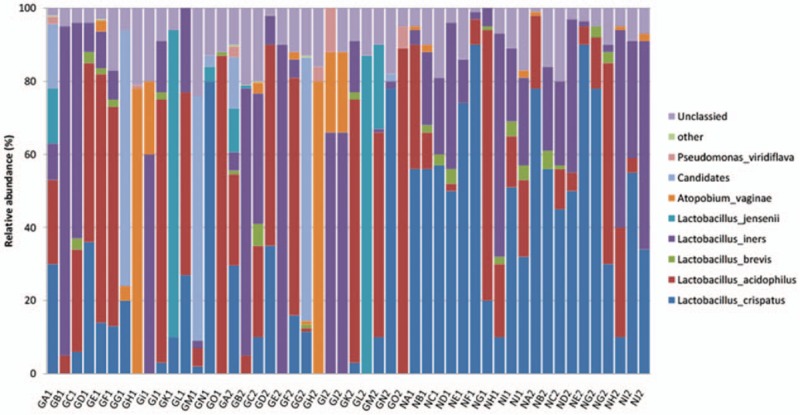
The taxonomic composition of the vaginal flora from the gestational diabetes mellitus (GDM) group (GA-GO) and the control group (NA-NJ) at the species level and based on 16S rRNA gene sequencing. For both groups, the samples of vaginal secretion collected at 28 to 30 weeks of pregnancy were numbered as “1” (e.g., GA1, NA1, ...), while those from 37 to 40 weeks of pregnancy were numbered as “2” (e.g., GA2, NA2, ...).
In the GDM group, the most abundant phylum was Firmicutes and the second was Proteobacteria (Fig. 1). In the control group, the most abundant phylum was also Firmicutes and the second was Proteobacteria (Fig. 1).
Lactobacillus was detected in most samples from both groups (Fig. 2). Lactobacillus was not detected in 1 patient with GDM during gestation, while Atopabium was the primary genus in this woman. In general, at the genus level, Lactobacillus remained the dominant genus during pregnancy. Over 96% of bacteria in vaginal flora were Lactobacillus, Corynebactrium, Achromobacter, Strepacoccus, Atopabium, Phyllobacterium, and Prevotelia. In the GDM group, 64 genera were detected in vaginal secretions at 28 to 30 weeks of pregnancy while 56 genera were detected at 37 to 40 weeks of pregnancy (Fig. 2). In the control group, only 36 genera were detected in vaginal secretions at 28 to 30 weeks of pregnancy while 34 genera were detected at 37 to 40 weeks of pregnancy (Fig. 2). The GDM group harbored 26 genera that were absent in the control group, and the control group had 2 unique genera.
Taxonomic analysis at the species level was conducted (Fig. 3). In the control group, the most abundant bacteria in vaginal flora was Lactobacillus crispatus, accounting for 28.5%; the second ones were Lactobacillus inersclone and Lactobacillus acidophilus, respectively, accounting for 20.9% and 13.7%. In the GDM group, the sequential order of Lactobacillus abundance in vaginal flora was: L acidophilus, 28.3%; L crispatus, 15.1%; and L inersclone, 12.8%. Other Lactobacillus species were also detected in vaginal secretions from both groups, but with lower abundance. The ratio of L jensenii was higher in the GDM group. In the GDM group, the composition of the vaginal flora also included Lactobacillus listeri, Lactobacillus amylovorus, and Lactobacillus fructivorans, which were absent in the vaginal secretions from healthy pregnant women. On the contrary, Lactobacillus salivarius was specific to healthy pregnant women.
4. Discussion
GDM is associated with adverse perinatal outcomes and with disturbances in the vaginal flora.[25–30] The association between candidiasis and adverse pregnancy outcomes is well known,[25–28] but less is known about the association between bacterial species and pregnancy outcomes.[31] Therefore, the present study aimed to examine the association between GDM and abnormal vaginal flora, and the association between abnormal vaginal flora and adverse pregnancy outcomes. The results strongly suggest that the vaginal infection rate was higher in GDM compared with healthy pregnant women. GDM and abnormal vaginal flora were both associated with adverse pregnancy outcomes. Vaginal Lactobacillus species were different between the 2 groups, which could contribute to the adverse outcomes.
The vaginal microecological environment is complex, it is affected by a variety of factors, and it is subject to dynamic changes.[17] In the present study, there was no significant difference regarding the frequency of BV between GDM patients and healthy pregnant women, but a significant difference was found regarding VVC. Pregnancy and DM are 2 independent causative factors for VVC.[29] VVC is a type of vaginitis caused by the overgrowth of the conditional pathogenic microorganism Candida, and it is one of the most common types of vaginitis.[17,20,22,25,38]Candida proliferates when the homeostasis of the vaginal microenvironment is disturbed, including changes in mucosal acidity and hormone levels. According to a previous study, the lifetime occurrence of at least 1 VVC event in healthy women of childbearing age is 75%, with recurrences in 50% of them.[39] During pregnancy, elevated hormone levels and glycogen accumulation in the vagina lead to a rise in VVC frequency, about 2 folds compared with nonpregnant women.[23,24,29–31,40] It is generally believed that pregnant women with GDM are predisposed to Candida colonization of the vagina.[25–28] Elevated glycemia in the vaginal tissue increases fungus adhesion and growth, predisposing the vaginal epithelial cells to binding to Candida albicans cells.[23,24] In addition, glycemia of 10 to 11 mmol/L could impair host defense mechanism, and hyperglycemia decreases neutrophil nonpurposeful migration and weakens their chemotactic and phagocytic powers, thereby elevating diabetic patient's sensitivity to VVC.[23,24]
Vaginal microecological abnormality is closely associated with adverse pregnancy (such as premature birth, PROM, and puerperal infection) and neonatal outcomes. VVC could cause retrograde infection, which gives rise to intrauterine infection, chorioamnionitis, and endometritis, causing PROM and abortion, premature birth, and intrauterine fetal death.[41] Gestational VVC without treatment can lead to vaginal injury during delivery, puerperal infection, and poor wound healing after perineal cut and cesarean section.[40] This is consistent with the present study since we also observed associations between abnormal vaginal flora and higher frequencies of PROM and chorioamnionitis or puerperal infection in healthy women, and with higher frequencies of PROM, premature delivery, and chorioamnionitis or puerperal infection in women with GDM.
Healthy women carry a variety of normal microorganisms in the vagina, of which Lactobacillus is the dominant bacteria, comprising 50 species, with a separation rate of 80% to 90%.[19] The present study showed that Lactobacillus was the dominant vaginal bacteria in both GDM and healthy pregnant women. The present study also found that the most abundant species among Lactobacillus was L crispatus in the vagina of healthy pregnant women, followed by L inersclone and L acidophilus, which is consistent with previous study results.[42–45] On the contrary, in the vagina of pregnant women with GDM, the descending order was L acidophilus, L crispatus, and L inersclone.
In vitro experiments by Mirmonsef et al[46] showed that the free glycogen content in vaginal secretions could affect pH values and colonization by various Lactobacillus species. Indeed, along with rising free glycogen levels in vaginal secretions, pH values decline and the levels of L crispatus and L jenseniiraise increase, while L inersclone remains unchanged.[46] Nevertheless, the present study revealed that the ratio of L crispatus in the vagina of pregnant women was 15.1% versus 28.5% in the GDM group versus healthy controls. Hence, L crispatus levels showed a decline, not an elevation, with descending vaginal pH values. This discrepancy could be attributed to a number of reasons, including differences in vitro versus in vivo and population-specific differences.
Vaginal Lactobacillus produces hydrogen peroxide to regulate microecological homeostasis, thereby preventing infections from external pathogens.[47,48] Lamont et al[49] reported that hydrogen peroxide was generated by 100% of L crispatus, but only by 80% of L acidophilus. The present study showed that even though the constitutional ratio of L acidophilus rose in the vagina of GDM pregnant women, the ratio of L crispatus declined, which could weaken the overall production of hydrogen peroxide, thereby favoring the growth of diverse conditional pathogens.
Compared with healthy pregnant women, the constitutional ratio of vaginal L inersclone was smaller in GDM pregnant women. Mirmonsef et al[46] revealed that the detection rate of vaginal L inersclone was 62.5% in healthy pregnant women, but only 8.3% in patients with gestational VVC. Thereby, L inersclone was proposed as a potential biomarker for vaginal microecological changes.[50] This decrease of L inersclone was also observed in GDM patients in the present study.
MacIntyre et al[51] showed that there was no significant difference in vaginal pH values or the detection rate of Lactobacillus during pregnancy. In the present study, irrespective of GDM or healthy pregnant women, the abundance and diversity of vaginal flora showed no significant difference between 28 to 30 and 37 to 40 weeks of pregnancy, and Lactobacillus was the dominant bacteria at both stages.
The present study is not without limitations. The sample size was small and from only 1 hospital. Only a few parameters were examined and inflammation parameters were not assessed.
In conclusion, the vaginal infection rate was higher in GDM compared with healthy pregnant women. GDM and abnormal vaginal flora were both associated with adverse pregnancy outcomes. Vaginal Lactobacillus species were different between the 2 groups, which could contribute to the adverse outcomes. The results of this study provide clues about the pathogenesis of GDM and associated conditions, as well as about potential targets for prophylaxis and treatment.
Author contributions
Data curation: Fengying Wang, Dan Li.
Formal analysis: Fengying Wang.
Writing – original draft: Xinhong Zhang.
Writing – review & editing: Qinping Liao.
Footnotes
Abbreviations: BV = bacterial vaginosis, GDM = gestational diabetes mellitus, IADPSG = the International Association of the Diabetes and Pregnancy Study Groups, OGTT = oral glucose tolerance test, VVC = vulvovaginal candidiasis.
The authors have no funding and conflicts of interest to disclose.
References
- [1].American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 2018;41(Suppl 1):S13–27. [DOI] [PubMed] [Google Scholar]
- [2].Agarwal MM, Dhatt GS, Shah SM. Gestational diabetes mellitus: simplifying the international association of diabetes and pregnancy diagnostic algorithm using fasting plasma glucose. Diabetes Care 2010;33:9.2018–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Werner EF, Pettker CM, Zuckerwise L, et al. Screening for gestational diabetes mellitus: are the criteria proposed by the international association of the Diabetes and Pregnancy Study Groups cost-effective? Diabetes Care 2012;35:529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhu WW, Yang HX, Wei YM, et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care 2013;36:586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sacks DA, Hadden DR, Maresh M, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 2012;35:526–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Duran A, Saenz S, Torrejon MJ, et al. Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos Gestational Diabetes Study. Diabetes Care 2014;37:2442–50. [DOI] [PubMed] [Google Scholar]
- [7].Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl 2):S251–60. [DOI] [PubMed] [Google Scholar]
- [8].Sacks DA, Black MH, Li X, et al. Adverse pregnancy outcomes using the international association of the diabetes and pregnancy study groups criteria: glycemic thresholds and associated risks. Obstet Gynecol 2015;126:67–73. [DOI] [PubMed] [Google Scholar]
- [9].Varner MW, Rice MM, Landon MB, et al. Pregnancies after the diagnosis of mild gestational diabetes mellitus and risk of cardiometabolic disorders. Obstet Gynecol 2017;129:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hirst JE, Tran TS, Do MA, et al. Consequences of gestational diabetes in an urban hospital in Viet Nam: a prospective cohort study. PLoS Med 2012;9:e1001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Waters TP, Dyer AR, Scholtens DM, et al. Maternal and neonatal morbidity for women who would be added to the diagnosis of GDM Using IADPSG criteria: a secondary analysis of the hyperglycemia and adverse pregnancy outcome study. Diabetes Care 2016;39:2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hawkins JS, Casey BM. Labor and delivery management for women with diabetes. Obstet Gynecol Clin North Am 2007;34:323–34. [DOI] [PubMed] [Google Scholar]
- [13].Kim SY, Sharma AJ, Sappenfield W, et al. Association of maternal body mass index, excessive weight gain, and gestational diabetes mellitus with large-for-gestational-age births. Obstet Gynecol 2014;123:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nesbitt TS, Gilbert WM, Herrchen B. Shoulder dystocia and associated risk factors with macrosomic infants born in California. Am J Obstet Gynecol 1998;179:476–80. [DOI] [PubMed] [Google Scholar]
- [15].Biggio JR, Jr, Chapman V, Neely C, et al. Fetal anomalies in obese women: the contribution of diabetes. Obstet Gynecol 2010;115(2 Pt 1):290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bental Y, Reichman B, Shiff Y, et al. Impact of maternal diabetes mellitus on mortality and morbidity of preterm infants (24-33 weeks’ gestation). Pediatrics 2011;128:e848–55. [DOI] [PubMed] [Google Scholar]
- [17].Vasquez A, Jakobsson T, Ahrne S, et al. Vaginal lactobacillus flora of healthy Swedish women. J Clin Microbiol 2002;40:2746–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sobel JD. Vulvovaginal candidosis. Lancet 2007;369:75–84. [DOI] [PubMed] [Google Scholar]
- [19].Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Leitich H, Bodner-Adler B, Brunbauer M, et al. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol 2003;189:139–47. [DOI] [PubMed] [Google Scholar]
- [21].Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG 2006;113(Suppl 3):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brocklehurst P, Gordon A, Heatley E, et al. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev 2013;1:CD000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mazziotti F, Arena V, Lo Mastro F, et al. Diabetes and pregnancy: prophylaxis of genital infections. Ann Ist Super Sanita 1997;33:343–5. [PubMed] [Google Scholar]
- [24].Nowakowska D, Kurnatowska A, Stray-Pedersen B, et al. Activity of hydrolytic enzymes in fungi isolated from diabetic pregnant women: is there any relationship between fungal alkaline and acid phosphatase activity and glycemic control? APMIS 2004;112:374–83. [DOI] [PubMed] [Google Scholar]
- [25].Hoosen AA, Peer AK, Seedat MA, et al. Vaginal infections in diabetic women: is empiric antifungal therapy appropriate? Sex Transm Dis 1993;20:265–8. [DOI] [PubMed] [Google Scholar]
- [26].Hirji I, Andersson SW, Guo Z, et al. Incidence of genital infection among patients with type 2 diabetes in the UK General Practice Research Database. J Diabetes Complications 2012;26:501–5. [DOI] [PubMed] [Google Scholar]
- [27].Rahman T, Khan IH, Begum J. High vaginal swab(HVS), routine microscopy and culture sensitivity in diabetic and non diabetic, a comparative retrospective study of five years. Indian J Med Sci 1991;45:212–4. [PubMed] [Google Scholar]
- [28].Guggenheimer J, Moore PA, Rossie K, et al. Insulin-dependent diabetes mellitus and oral soft tissue pathologies: II. Prevalence and characteristics of Candida and Candidal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;89:570–6. [DOI] [PubMed] [Google Scholar]
- [29].Nowakowska D, Kurnatowska A, Stray-Pedersen B, et al. Prevalence of fungi in the vagina, rectum and oral cavity in pregnant diabetic women: relation to gestational age and symptoms. Acta Obstet Gynecol Scand 2004;83:251–6. [DOI] [PubMed] [Google Scholar]
- [30].Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol 2016;42:905–27. [DOI] [PubMed] [Google Scholar]
- [31].Marschalek J, Farr A, Kiss H, et al. Risk of vaginal infections at early gestation in patients with diabetic conditions during pregnancy: a retrospective cohort study. PLoS One 2016;11:e0155182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zeng Z, Pan L, Zhou D. [Clinical microecology and the theory principle]. Chin J Microecol 1999;12:321–31. [Google Scholar]
- [33].Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991;29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fadrosh DW, Ma B, Gajer P, et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011;27:2957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 2013;10:996–8. [DOI] [PubMed] [Google Scholar]
- [37].Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Goldenberg RL, Culhane JF, Johnson DC. Maternal infection and adverse fetal and neonatal outcomes. Clin Perinatol 2005;32:523–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mardh PA, Rodrigues AG, Genc M, et al. Facts and myths on recurrent vulvovaginal candidosis – a review on epidemiology, clinical manifestations, diagnosis, pathogenesis and therapy. Int J STD AIDS 2002;13:522–39. [DOI] [PubMed] [Google Scholar]
- [40].Ibara AS, Marcorelles P, Le Martelot MT, et al. Two cases of systemic Candida glabrata infection following in vitro fertilization and embryo transfer. Eur J Clin Microbiol Infect Dis 2004;23:53–6. [DOI] [PubMed] [Google Scholar]
- [41].Kiss H, Petricevic L, Husslein P. Prospective randomised controlled trial of an infection screening programme to reduce the rate of preterm delivery. BMJ 2004;329:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Martinez-Pena MD, Castro-Escarpulli G, Aguilera-Arreola MG. Lactobacillus species isolated from vaginal secretions of healthy and bacterial vaginosis-intermediate Mexican women: a prospective study. BMC Infect Dis 2013;13:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis 1999;180:1950–6. [DOI] [PubMed] [Google Scholar]
- [44].Zhang R, Daroczy K, Xiao B, et al. Qualitative and semiquantitative analysis of Lactobacillus species in the vaginas of healthy fertile and postmenopausal Chinese women. J Med Microbiol 2012;61(Pt 5):729–39. [DOI] [PubMed] [Google Scholar]
- [45].Yan DH, Lu Z, Su JR. Comparison of main lactobacillus species between healthy women and women with bacterial vaginosis. Chin Med J (Engl) 2009;122:2748–51. [PubMed] [Google Scholar]
- [46].Mirmonsef P, Hotton AL, Gilbert D, et al. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PLoS One 2014;9:e102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1beta, independent of bacterial vaginosis. Sex Transm Dis 2015;42:358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Borges S, Silva J, Teixeira P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet 2014;289:479–89. [DOI] [PubMed] [Google Scholar]
- [49].Lamont RF, Sobel JD, Akins RA, et al. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG 2011;118:533–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jakobsson T, Forsum U. Lactobacillus iners: a marker of changes in the vaginal flora? J Clin Microbiol 2007;45:3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].MacIntyre DA, Chandiramani M, Lee YS, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep 2015;5:8988. [DOI] [PMC free article] [PubMed] [Google Scholar]


