Abstract
This study aims to explore the roles of cysteine-rich protein 61 (Cyr61/CCN1), connective tissue growth factor (CTGF/CCN2) and vascular endothelial growth factor (VEGF) in the vascular process of polymyositis (PM)/dermatomyositis (DM).
Real-time quantitative polymerase chain reaction was used to determine the mRNA expression of Cyr61, CTGF, and VEGF in muscle tissues of initially treated PM/DM patients and controls. Enzyme-linked immunosorbent assay (ELISA) was used to determine the serum levels of Cyr61, CTGF, and VEGF of initially treated PM/DM patients before and after treatment. Data were statistically analyzed using statistical software SPSS 17.0.
The mRNA expression levels of Cyr61, CTGF, and VEGF in muscle tissues were higher in the PM and DM groups than in the control group (P < .05). Differences in the mRNA expression levels of Cyr61, CTGF, and VEGF in muscle tissues between the PM and DM groups were not statistically significant (P > .05). Before treatment, the serum levels of Cyr61, CTGF, and VEGF were higher in the PM and DM groups than in the control group (P < .05). Furthermore, in the PM and DM groups, the expression levels of Cyr61, CTGF, and VEGF in serum at 6 months after treatment were lower than those before treatment (P < .05).
Cyr61, CTGF, and VEGF are involved in the pathogenesis of PM/DM. These may be involved in the pathogenesis mainly by affecting the formation of blood vessels and promoting inflammatory response. This suggests that microvascular lesions play an important role in the immune pathogenesis of inflammatory myopathy PM/DM.
Keywords: CTGF, Cyr61, polymyositis/dermatomyositis, RT-qPCR, VEGF
1. Introduction
Polymyositis (PM)/dermatomyositis (DM) is a nonsuppurative inflammatory myopathy characterized by invasion of the skeletal muscle, which affects multiple organs and systems, and even endangers the life of the patient when it becomes severe. It is a common autoimmune disease, and its 10-year mortality reaches up to 26.3%. However, its pathogenesis remains unknown. In recent years, attention has been given to the role of vascular factors in the pathogenesis of the disease. It has been considered that PM/DM is a kind of microangiopathy. The pathological changes of PM/DM are caused by the deposition of the immune complex on the vascular wall or direct damage to vascular endothelial cells. However, in addition to this, it remains unknown whether other factors that can cause vascular diseases are present. CCN protein family members, cysteine-rich protein 61 (Cyr61/CCN1) and connective tissue growth factor (CTGF/CCN2), have similar structures, and are rich in cysteine. Cyr61 is an important angiogenic regulator.[1] In the process of bone formation and embryo development, CTGF plays an important role in the formation of cartilage and angiogenesis.[2,3] Vascular endothelial growth factor (VEGF) plays an important role in regulating angiogenesis.[4,5] Therefore, in the present study, real-time quantitative polymerase chain reaction (RT-qPCR) was used to determine the mRNA expression of Cyr61, CTGF, and VEGF in muscle tissues of initially treated PM/DM patients, and enzyme-linked immunosorbent assay (ELISA) was used to determine the serum levels of Cyr61, CTGF, and VEGF of initially treated PM/DM patients before and after treatment, in order to explore the roles of Cyr61, CTGF, and VEGF in the vascular process of PM/DM, and further explore the possible pathogenesis of PM/DM.
2. Subjects and methods
2.1. Study subjects
The mRNA expression of Cyr61, CTGF, and VEGF in muscle tissues obtained from the subjects was determined by RT-qPCR.
2.1.1. PM group
A total of 17 PM patients, who were admitted to the Department of Rheumatology of the Affiliated Hospital of Qinghai University from March 2015 to September 2016, were included into this group. Among these patients, 7 patients were male and 10 patients were female, and the age of these patients ranged within 22 to 62 years, with an average age of 43.5 ± 14.1 years.
2.1.2. DM group
A total of 18 DM patients, who were admitted to the Department of Rheumatology of the Affiliated Hospital of Qinghai University from March 2015 to September 2016, were included into this group. Among these patients, 7 patients were male and 11 patients were female, and the age of these patients ranged within 23 to 71 years, with an average of 43.5 ± 11.1 years.
2.1.3. Control group
A total of 20 healthy subjects with matched age and sex in the Medical Examination Center of the Affiliated Hospital of Qinghai University were selected as controls. Among these subjects, 4 subjects were male and 16 subjects were female. The age of these subjects ranged within 17 to 67 years, with an average age of 37.1 ± 12.0 years. Patients with PM/DM were excluded from the control group.
2.2. Diagnostic criteria
All PM/DM patients met the PM/DM diagnostic criteria recommended by Bohan and Peter (B/P criteria).
2.3. Exclusion criteria
Patients with a history of primary heart, lung, liver and kidney diseases, as well as other diseases, and patients who recently had severe infection, myasthenia caused by other causes, tumors, and other rheumatoid immune diseases, were all excluded in the disease groups and control group.
The differences in sex and age among the control group, DM group, and PM group were not statistically significant (P > .05).
The present study was approved by the Ethics Committee of our hospital. All subjects provided an informed consent before enrollment into this study.
2.4. Specimen acquisition and processing
All patients underwent muscle biopsy. Tissues from more seriously affected muscles, such as the musculus triceps brachii or quadriceps femoris, were collected. Normal muscle tissues near the orthopedic trauma site were collected and assigned to the control group. The volume of these specimens was approximately 0.5 × 0.5 × 1 cm. After collection, these specimens were placed in saline and blood was emptied. Then, the specimen was placed in an EP tube, and marked and placed in the refrigerator at −80°C for RT-qPCR detection.
From each subject, 5 mL of fasting venous blood was collected in the early morning as serum samples, which were placed in a blood donation tube with coagulant. Within 2 hours, the blood sample was centrifuged at 3000 rpm for 15 minutes. Then, the stratified serum was extracted, placed in the EP tube, and subsequently placed in the refrigerator at −80 °C for ELISA.
RT-qPCR was used to determine the mRNA expression of Cyr61, CTGF, and VEGF in muscle tissues.
Total RNA was extracted from muscle tissues in the 2 groups by Trizol protocol. The total RNA concentration was measured in triplicate using a UV spectrophotometer, and the A260/280 was measured to be 1.8 to 2.0. Furthermore, cDNA was synthesized through reverse transcription according to reagent instructions, and was used as a template to undergo PCR amplification in a 20-μL system, according to the instructions of the RT-PCR amplification kit.
GAPDH was used as the reference gene, the internal reference gene and the target gene were replicated 3 times, the Ct values were averaged, and the Delta Ct method was used to evaluate the difference in gene expression levels between the groups; the gene expression change folds were compared using the 2-ΔΔCt method; ΔΔCt Method: A = CT (target gene, sample to be tested)–CT (internal standard gene, sample to be tested), B = CT (target gene, control sample)–CT (internal standard gene, control sample), K = AB, Expression multiple = 2K.
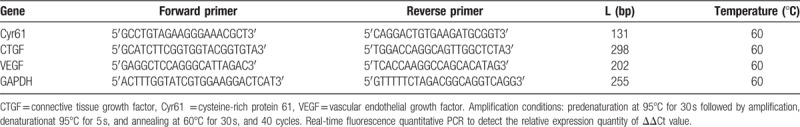
Sequences of the target gene primers:
The primers were designed and synthesized by Invitrogen. The primer sequences are shown in Table 1.
Table 1.
The sequence of the primer of Cyr61, CTGF, and VEGF.
ELISA was used to determine the serum levels of Cyr61, CTGF, and VEGF in PM/DM patients before treatment and at six months after treatment
The human Cyr61, CTGF, and VEGF ELISA kits were provided by Shanghai Jingke Industrial Co., Ltd. In this experiment, the serum levels of Cyr61, CTGF, and VEGF were determined according to the instructions of Cyr61, CTGF, and VEGF ELISA kits.
2.5. Statistical analysis
Data were statistically analyzed using SPSS 21.0 software. Measurement data were expressed as mean ± standard deviation (x ± SD), compared between 2 groups using independent sample t test, and compared among 3 groups using univariate analysis of variance. The inspection level was set at α = 0.05.
3. Results
3.1. RT-qPCR results
In the PM group, the Cyr61 mRNA level in the muscle tissue was 15.21 ± 6.88, the CTGF mRNA level was 13.08 ± 7.04, and the VEGF mRNA level was 15.57 ± 6.49. The Cyr61 mRNA level in the muscle tissue of the DM group was 17.77 ± 13.32, the CTGF mRNA level was 12.35 ± 7.27, and the VEGF mRNA. The level was 17.70 ± 7.20. In the control group, the Cyr61 mRNA level in the muscle tissue was 1.79 ± 0.66, the CTGF mRNA level was 1.70 ± 0.80, and the VEGF mRNA level was 1.50 ± 0.77.
The mRNA expression levels of Cyr61, CTGF, and VEGF in muscle tissues were higher in the PM and DM groups than in the control group (P < .05), and the difference between the PM and DM groups was not statistically significant (P > .05, Table 2).
Table 2.
Comparison of the mRNA expression levels of Cyr61, CTGF, and VEGF in muscle tissues among the 3 groups 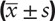 .
.

3.2. ELISA results
In the PM group, the serum Cyr61 level was 1298.70 ± 157.28, the CTGF level was 1235.60 ± 437.30, and the VEGF level was 157.80 ± 50.27. The serum Cyr61 level in the DM group was 1235.10 ± 205.48, the CTGF level was 1212.20 ± 273.75, and VEGF level before the treatment was 190.29 ± 26.04. In the control group, the serum Cyr61 level was 808.80 ± 215.90, the CTGF level was 720.05 ± 159.7, and the VEGF level was 87.97 ± 32.53.
Before treatment, the serum levels of Cyr61, CTGF, and VEGF were higher in the PM and DM groups than in the control group (P < .05). In the PM and DM groups, the expression levels of Cyr61, CTGF, and VEGF in serum at 6 months after treatment were lower than those before treatment (P < .05, Table 3).
Table 3.
Comparison of the expression level ofCyr61, CTGF, and VEGF in serum among the 3 groups 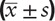 .
.

4. Discussion
The pathogenesis of PM/DM was closely correlated to the mechanism of autoimmunity. Previous studies have revealed that PM was mainly induced by cellular immunity, and DM was mainly induced by humoral immunity. Microvascular involvement was first discovered in DM, and was also found in PM. Furthermore, the expression of adhesion molecules and proinflammatory factors were elevated in endothelial cells in both PM and DM patients. Studies have revealed that in PM patients, the expression of intercellular adhesion molecule-1 and interleukin-1 were significantly upregulated, and most of the microvascular structures were abnormal under an electron microscope. These all suggest that vascular and inflammatory factors may be involved in the pathogenesis of PM/DM.[6,7]
Angiogenesis requires the induction by a variety of growth factors. The most important known positive regulatory factor is VEGF. As one of the most common and effective vascular growth-promoting cytokines, VEGF can bind with the VEGF receptor to activate the intracellular signaling cascade pathway, accelerate the proliferation of endothelial cells, and increase angiogenesis.[8] VEGF is the most effective angiogenesis growth factor.[9] A study revealed that a variety of mechanisms are found beyond the VEGF-related angiogenesis of tumors.[10] Hypoxia is one of the most important regulatory factors for the expression of VEGF. In the present study, RT-qPCR results revealed that the mRNA expression levels of Cyr61, CTGF, and VEGF in muscle tissues were higher in the PM and DM groups than in the control group. This suggests that Cyr61, CTGF, and VEGF may be involved in the pathogenesis of PM/DM. A study revealed that there was hypoxia in the muscle tissues of PM/DM patients. Furthermore, there were a number of pathogenic autoantibodies, activated complements, immune-complexes, and cytokines (such as interleukin-1 [IL-1], tumor necrosis factor [TNF], among others) in the body of PM/DM patients. A study revealed that inflammatory factors such as IL-1, TNF-α, bacteria and, viruses can induce the expression of Cyr61, whereas Cyr61 can aggravate the inflammatory response.[11] Another study revealed that the level of CCN1 was elevated in patients with some chronic inflammatory diseases, including colitis and rheumatoid arthritis.[12] The overexpression of Cyr61 can promote the growth, migration, adhesion, and survival of vascular endothelial cells, and enhance the activity of VEGF and other growth factors, to promote the growth of tumor vessels. It has been found that CTGF can be produced by various types of cells, such as fibroblasts, endothelial cells, vascular smooth muscle cells, glomerular membrane cells, osteoblasts, and chondrocytes. Some studies have concluded that CTGF may have the potential to stimulate the angiogenesis of glioma and function of angiogenic factors. Furthermore, CTGF plays an important role in the formation of cartilage and angiogenesis in the process of bone formation and embryo development.[2,3] A study revealed that CTGF is very important for the extracellular matrix (ECM) and embryo angiogenesis. At the molecular level, CTGF/CCN2 can stimulate the transformation of differentiated cells, such as tubular epithelial cells, endothelial cells and fibroblasts.[13–16] When CTGF is activated by transforming growth factor-β (TGF-β) at the transcriptional level, it stimulates the proliferation of connective tissue cells and the synthesis of ECM, and promotes fibrosis and angiogenesis.[2] CTGF has a natural and powerful angiogenic activity. An experiment revealed that as the expression of CTGF increased, the mRNA and protein levels of VEGF also correspondingly increased. Thus, the expression of these two demonstrates a dose-dependent correlation. It was speculated that CTGF may contribute to the formation of new blood vessels of tumors by promoting the expression of VEGF. In addition, CTGF may work in chemical chemotaxis, promote the migration of vascular endothelial cells, and increase angiogenic activity. In the present study, ELISA revealed that before treatment, the serum levels of Cyr61, CTGF, and VEGF were higher in the PM and DM groups than in the control group. Furthermore, in the PM and DM groups, the expression levels of Cyr61, CTGF, and VEGF in serum at 6 months after treatment were lower than those before treatment, and the differences were statistically significant (P < .05). These further reveal that Cyr61, CTGF, and VEGF are involved in the pathogenesis of PM/DM, which decreases with the improvement of the condition. Therefore, it could be considered that in local muscle tissues of PM/DM patients, inflammation and hypoxia promote the expression of VEGF, inflammation promotes the expression of Cyr61, and elevated Cyr61 levels would also promote the increase in VEGF production, further promoting angiogenesis. Similarly, this inflammatory reaction can activate CTGF. In addition to directly promoting angiogenesis, Cyr61 and CTGF can also indirectly promote angiogenesis by increasing the expression of vascular growth regulator factors, such as VEGF. As the condition of PM/DM patients improves, the expression of angiogenic factors VEGF, Cyr61, and CTGF gradually decreases. This suggests that microvascular lesions play an important role in the immune pathogenesis of inflammatory myopathy PM/DM.
Studies on the role of angiogenic factors VEGF, Cyr61, and CTGF in the pathogenesis of PM/DM would be helpful in further studying the pathogenesis, prevention, and treatment of PM/DM. However, PM/DM is a complex autoimmune disease, and many other factors are involved in its pathogenesis. Furthermore, the number of cases in this study was small. Therefore, further research and discussions are needed.
Author contributions
Conceptualization: Kexia Chai.
Data curation: Yuqi Chen, Peilin Fan.
Formal analysis: Yuqi Chen, Peilin Fan.
Funding acquisition: Kexia Chai.
Investigation: Jie Yang, Xia Yuan.
Methodology: Yuqi Chen, Peilin Fan.
Project administration: Kexia Chai.
Software: Yuqi Chen, Peilin Fan.
Writing – original draft: Kexia Chai.
Writing – review & editing: Kexia Chai.
Footnotes
Abbreviations: B/P criteria = Bohan and Peter, CTGF/CCN2 = connective tissue growth factor, Cyr61/CCN1 = cysteine-rich protein 61, DM = dermatomyositis, ECM = extracellular matrix, ELISA = enzyme-linked immunosorbent assay, PM = polymyositis, RT-qPCR = real-time quantitative polymerase chain reaction, TGF-β = transforming growth factor-β, VEGF = vascular endothelial growth factor.
This study was supported by Qinghai Provincial Science and Technology Department Applied Basic Research Project (2015-ZJ-756).
The authors report no conflicts of interest.
References
- [1].Mo FE, Muntean AG, Chen CC, et al. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Bio 2002;22:8709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rachfal AW, Brigstock DR. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol Res 2003;26:1–9. [DOI] [PubMed] [Google Scholar]
- [3].Kurikawa N, Suga M, Kuroda S, et al. An angiotensin II type 1 receptor antagonist, olmesartan medoxomil, improves experimental liver fibrosis by suppression of proliferation and collagen synthesis in activated hepatic stellate cells. Br J Pharmacol 2003;139:1085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tsai MS, Bogart DF, Li P, et al. Expression and regulation of Cyr61 in human breast cancer cell lines. Oncogene 2002;21:964–73. [DOI] [PubMed] [Google Scholar]
- [5].Moon WS, Rhyu KH, Kang MJ, et al. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Mod Pathol 2003;16:552–7. [DOI] [PubMed] [Google Scholar]
- [6].De Letter MA, van Doorn PA, Savelkoul HF, et al. Critical illness polyneuropathy and myopathy (CIPNM): evidence for local immune activation by cytokine-expression in the muscle tissue. J Neuroimmunol 2000;106:206–13. [DOI] [PubMed] [Google Scholar]
- [7].Ito T, Kumamoto T, Horinouchi H, et al. Adhesion molecule expression in experimental myositis. Muscle Nerve 2002;25:409–18. [DOI] [PubMed] [Google Scholar]
- [8].Abdel-Rahman O. Targeting vascular endotheial growth factor (VEGF) pathway in gastric cancer: preclinical and clincal aspects. Crit Rev Oncol Hematol 2015;93:18–27. [DOI] [PubMed] [Google Scholar]
- [9].Ahluwalia A, Jones MK, Matysiak-Budnik T, Tarnawski AS. VEGF and colon cancer growth beyond angiogenesis: does VEGF directly mediate colon cancer growth via a non-angiogenic mechanism? Curr Pharm Des 2014;20:1041–4. [DOI] [PubMed] [Google Scholar]
- [10].Dong J, Saunders D, Silasi-Mansat R, et al. Therapeutic efficacy of a synthetic epsin mimetic peptide in glioma tumor model: uncovering multiple mechanisms beyond the VEGF-associated tumor angiogenesis. J Neurooncol 2018;138:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bai T, Chen CC, Lau LF. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol 2010;184:3223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Krupska I, Bruford EA, Chaqour B. Eyeing the Cyr61/CTGF/NOV (CCN) group of genes in development and diseases:highlights of their structural likenesses and functional dissimilarities. Hum Genomics 2015;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Klenotic PA, Zhang C, Lin Z. Emerging roles of CCN proteins in vascular development and pathology. J Cell Commun Signal 2016;10:251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abu El-Asrar AM, De Hertogh G, van den Eynde K, et al. Myofibroblasts in proliferative diabetic retinopathy can originate from infiltrating fibrocytes and through endothelial-to-mesenchymal transition (EndoMT). Exp Eye Res 2015;132:179–89. [DOI] [PubMed] [Google Scholar]
- [15].Dockrell ME, Phanish MK, Hendry BM. Tgf-beta auto-induction and connective tissue growth factor expression in human renal tubule epithelial cells requires N-ras. Nephron Exp Nephrol 2009;112:e71–9. [DOI] [PubMed] [Google Scholar]
- [16].Sonnylal S, Xu S, Jones H, et al. Connective tissue growth factor causes EMT-like cell fate changes in vivo and in vitro. J Cell Sci 2013;126:2164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


