Abstract
Background
Medicaid insurance in Georgia provides limited reimbursement for heart transplant (HT) and left ventricular assist devices (LVAD). We examined whether insurance type affects eligibility for and survival after receipt of HT or LVAD.
Methods and Results
We retrospectively identified patients evaluated for HT/LVAD from 2012 to 2016. We used multivariable logistic and Cox proportional hazards regression to examine the association of insurance type on treatment eligibility and one year survival. Of 569 patients evaluated, 282 (49.6%) had private, 222 (39.0%) had Medicare, and 65 (11.4%) had Medicaid insurance. Patients with Medicaid were younger, more likely to be Black, with fewer medical comorbidities. In adjusted models, Medicare and Medicaid insurance predicted lower odds of eligibility for HT, but did not affect survival after HT. Among those ineligible for HT, Medicaid patients were less likely to receive destination therapy (DT) LVAD (adj OR 0.08, 95% CI 0.01–0.66; P=0.02) and had increased risk of death (adj HR=2.03, 95% CI 1.13 –3.63; p=0.01).
Conclusions
Despite younger age and fewer comorbidities, patients with Medicaid insurance are less likely to receive DT LVAD, and have an increased risk of death once deemed ineligible for HT. Medicaid patients in Georgia need improved access to DT LVAD.
Keywords: heart transplantation, left ventricular assist device, health disparities, healthcare policy, insurance
INTRODUCTION
More than 100,000 Americans with end-stage heart failure (HF) are refractory to optimal medical therapy, with a 1-year survival of only 10% to 25%.1–3 Heart transplant (HT) and left ventricular assist devices (LVAD) have dramatically changed the management and prognosis of patients with end-stage HF. HT remains the therapy of choice, with a median conditional survival that now exceeds thirteen years4, and substantial improvements in quality of life and functional status compared to medical therapy.4 The demand for HT, however, far outpaces the supply of donor organs.5 Surgical treatment of HF with a LVAD has become standard of care to clinically stabilize patients who deteriorate while awaiting HT and to improve survival and quality of life in some patients ineligible for HT.6,7
Evaluation to determine eligibility for HT or LVAD is a rigorous process that thoroughly evaluates all medical comorbidities, as well as psychosocial and/or socioeconomic factors that would identify candidates at high risk for poor outcomes. Eligibility for HT or LVAD are determined using specific criteria endorsed by international organizations8,9, in an effort to provide guidance to individual centers. All noncardiac medical conditions are assessed that may limit survival independent of heart disease (i.e. advanced renal or hepatic dysfunction, uncontrolled diabetes, peripheral vascular disease, etc.). In addition, psychosocial and socioeconomic evaluation is recommended to assess the patient’s available support systems to achieve maximal compliance with medical care after the HT or LVAD surgery.
Multiple prior studies have identified public insurance as a risk factor for inferior outcomes after HT, likely related to differences in access to health care providers and the costs of immunosuppressant medications.10–13 Prior data has confirmed that very few uninsured or underinsured patients receive HT in the United States (US), as most HT programs require adequate insurance coverage and financial resources to list patients for transplantation.14,15 Currently, little data exists on the impact that insurance has on outcomes after LVAD. Moreover, since national databases that track HT and LVAD outcomes do not capture data on all patients evaluated for those therapies, it is very difficult to know how insurance impacts access to or eligibility for advanced HF therapies.
Currently, Medicaid insurance in the state of Georgia does not provide reimbursement for HT for beneficiaries over the age of 21, and provides limited reimbursement for LVAD. Historically, our hospital has offered HT (and LVAD in rare instances) to adult Medicaid patients through hospital charity care. However, increasing financial pressures on hospital systems place these services at risk for cutbacks. Currently, there is no data investigating whether these policies are associated with disparities in access based on insurance type. Thus, the objectives of this retrospective cohort analysis are to 1) describe the sociodemographic and clinical characteristics of patients with end-stage HF evaluated for HT or LVAD according to insurance type at our center to compare the medical, financial, and psychosocial comorbidities according to insurance type, and 2) examine the clinical outcomes of patients according to insurance type after eligibility for HT or LVAD has been determined..
METHODS
Study population
We retrospectively examined all patients evaluated for advanced HF therapies at Emory University Hospital from January 1, 2012 to December 31, 2016 (N=574). Patients who had previously received HT and were evaluated for re-transplant during this period were excluded from the study (N=5). Eligibility for HT or LVAD is determined using criteria specified in the Emory University Hospital Guidelines for Recipient Candidacy for HT and LVAD. Decisions regarding eligibility are made by an advanced HF therapeutics committee, which includes HF/transplant cardiologists and surgeons, HT and LVAD nurse coordinators, biomedical engineers, a pharmacist, social workers, financial counselors, dieticians, and a physical therapist. All patients evaluated for HT and LVAD are recorded in the Emory HT database, and all decisions made regarding final candidacy for HT or LVAD are documented in the medical record. Transplant centers are required to provide a letter to all patients evaluated for HT that documents the specific reasons that a patient is not considered a HT candidate; these reasons are documented in the medical record. This study was approved by the Emory University Institutional Review Board.
Study endpoints
The primary endpoint for this analysis was eligibility for HT/LVAD, while the secondary endpoint was survival at one year from the date of initial evaluation. Those candidates who meet the specified criteria will be “listed” for HT, while those candidates who do not meet the specified criteria for HT will be considered for DT LVAD or considered ineligible for advanced HF therapies. Outcomes of advanced therapy evaluations and reasons for ineligibility were determined by medical record review. Survival was determined by medical record review and/or Social Security Death Index query. Patients were censored at the time of loss to follow-up or at the last date of follow-up on October 1, 2017.
Study covariates
Information on demographic, psychosocial, socioeconomic, and medical covariates was documented at the time of the HT/LVAD evaluation. The primary exposure of interest was defined as insurance type at the time of evaluation (private, Medicare, or Medicaid). Patients with Medicare and Medicaid coverage (N=31) were classified as having Medicare. Covariates of interest included the following variables: age, gender, HF etiology, history of hypertension, history of diabetes mellitus (DM), history of coronary artery disease (CAD), history of chronic kidney disease (CKD), history of left ventricular (LV) thrombus, requirement for home inotropes, body mass index (BMI), serum sodium, estimated glomerular filtration rate (eGFR), serum albumin, serum total bilirubin, panel reactive antibodies (PRA), pulmonary capillary wedge (PCW) pressure, right atrial (RA) pressure and cardiac index (CI). Specific reasons that a patient was not considered to be a HT or LVAD candidate were also considered as covariates, including advanced age (age ≥ 70 years, or ≥65 years if being evaluated for dual organ transplant), medical comorbidities (calculated panel reactive antibody ≥ 75%, diabetes with end organ complications, advanced liver disease, end stage renal disease, severe lung disease, etc), inadequate social support (lack of a primary caregiver who is able to meet the patients’ needs post-transplant or post-LVAD implantation), inadequate financial resources (unable to afford the cost of medication co-payments in the first year post-transplant or post-LVAD implantation), medical noncompliance (history of a persistent pattern of refusal to take prescribed medications, come to regularly scheduled outpatient appointments, or follow a prescribed course of treatment), and active substance abuse (abuse of alcohol, cigarettes, or illicit substances within the 6 months prior to evaluation for HT).
Statistical analysis
Data are presented as mean ± standard deviation (SD) or N (%) of patients. Baseline characteristics were compared between patients according to insurance using one-way analysis of variance for continuous variables and the χ2 or Fisher exact test for categorical variables. Differences in outcomes of the evaluation (HT, DT LVAD or ineligible) and reasons for ineligibility by insurance type were compared using the χ2 or Fisher exact test. The association of insurance type with eligibility for HT and DT LVAD was examined using multivariable logistic regression, adjusted for age, sex, race, HF etiology, BMI, CKD stage, serum albumin, dependence on continuous inotropes, and the reasons for ineligibility for HT or LVAD. The association of insurance type on survival at one year was examined using Cox proportional hazards regression, adjusted for age, sex, race, HF etiology, BMI, CKD stage, serum albumin and dependence on continuous inotropes. We constructed survival curves adjusted for the group-specific mean of these covariates. Survival analysis was performed for all patients and then in groups stratified by patient outcome (listed for HT vs. not listed). As an exploratory analysis, the Fine and Gray model of competing risks (with transplant being treated as a competing event), was used to estimate the risk of death according to insurance type in those patients who were listed for HT.16 Data were analyzed with the use of SAS statistical software version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Baseline characteristics
During the study period, 569 patients were evaluated for primary HT and/or LVAD implantation. The baseline characteristics of the cohort are displayed in Table 1. The mean age was 51.7 ± 12.7 years, 391 (68.7%) patients were male, and 307 (54.0%) were Black. Compared to patients with private or Medicare insurance, patients with Medicaid insurance were younger and more likely to be Black. Medicaid patients had fewer medical comorbidities, including better renal function, lower BMI, and were less likely to have CAD and hypertension. There was no difference by insurance type in inotrope dependence or hemodynamics (including RA, PCW and CI) at evaluation.
Table 1.
Baseline characteristics of the 569 patients evaluated for HT/LVAD from 2012–2016 according to insurance type.
| Private N=282 |
Medicare N=222 |
Medicaid N=65 |
P-value | |
|---|---|---|---|---|
|
| ||||
| Age, years | 50.5±11.5 | 56.2±12.1 | 42.0±13.6 | <0.0001 |
|
| ||||
| Race | 0.0007 | |||
| • White | 134 (47.5) | 92 (41.4) | 13 (20.0) | |
| • Black | 135 (47.9) | 123 (55.4) | 49 (75.4) | |
| • Other | 13 (4.6) | 7 (3.2) | 3 (4.6) | |
|
| ||||
| Male | 199 (70.6) | 149 (67.1) | 43 (66.2) | 0.6 |
|
| ||||
| Heart failure etiology | 0.009 | |||
| • Ischemic | 65 (23.1) | 73 (32.9) | 15 (23.1) | |
| • Nonischemic | 170 (60.3) | 122 (55.0) | 30 (46.2) | |
| • Peripartum | 10 (3.6) | 7 (3.2) | 8 (12.3) | |
| • Restrictive | 2 (0.7) | 0 (0) | 0 (0) | |
| • ACHD | 7 (2.5) | 5 (2.3) | 3 (4.6) | |
| • Other | 28 (9.9) | 15 (6.8) | 9 (13.8) | |
|
| ||||
| Coronary artery disease | 123 (43.6) | 116 (52.3) | 21 (32.3) | 0.01 |
|
| ||||
| Hypertension | 170 (60.3) | 140 (63.1) | 30 (46.2) | 0.04 |
|
| ||||
| Diabetes mellitus | 84 (29.8) | 92 (41.4) | 21 (32.3) | 0.02 |
|
| ||||
| Lung Disease | 28 (9.9) | 34 (15.3) | 9 (13.9) | 0.2 |
|
| ||||
| Left ventricular thrombus | 39 (13.8) | 26 (11.7) | 14 (21.5) | 0.1 |
|
| ||||
| Peripheral arterial disease | 3 (1.1) | 13 (5.9) | 0 (0 | 0.002 |
|
| ||||
| Chronic kidney disease stage | 0.001 | |||
| • 1–2 | 130 (46.0) | 85 (38.3) | 42 (64.6) | |
| • 3 | 122 (43.2) | 115 (51.8) | 21 (32.3) | |
| • 4 | 28 (9.9) | 19 (8.6) | 2 (3.1) | |
| • 5 | 2 (0.7) | 3 (1.4) | 0 (0.0) | |
|
| ||||
| Eval for dual organ transplant | 0.1 | |||
| • Heart-kidney | 33 (11.7) | 29 (13.1) | 3 (4.6) | |
| • Heart-liver | ||||
|
| ||||
| Inotrope Dependent | 170 (60.3) | 144 (64.9) | 42 (64.6) | 0.6 |
|
| ||||
| INTERMACS profile | 0.01 | |||
| • 1–2 | 41 (14.5) | 33 (14.9) | 20 (30.8) | |
| • 3 | 129 (45.7) | 111 (50.0) | 22 (33.8) | |
| • 4–7 | 112 (39.7) | 78 (35.1) | 23 (35.4) | |
|
| ||||
| Blood type | 0.6 | |||
| • O | 118 (44.4) | 98 (47.3) | 31 (50.8) | |
| • A | 91 (34.2) | 72 (34.8) | 13 (21.3) | |
| • B | 45 (16.9) | 28 (13.5) | 12 (19.7) | |
| • AB | 12 (4.5) | 9 (4.3) | 5 (8.2) | |
|
| ||||
| Body mass index, kg/m2 | 29.3±6.6 | 29.7±7.4 | 26.6±5.8 | 0.007 |
|
| ||||
| Albumin, g/dL | 3.5±0.6 | 3.4±0.5 | 3.4±0.7 | 0.4 |
|
| ||||
| Total bilirubin, mg/dL | 1.6±1.3 | 1.6±1.1 | 1.8±1.3 | 0.5 |
|
| ||||
| Creatinine, mg/dL | 1.5±0.7 | 1.6±0.8 | 1.4±0.5 | 0.06 |
|
| ||||
| eGFR, ml/min/1.73 m2 | 60.0±24.1 | 55.2±22.1 | 68.8±26.8 | 0.0002 |
|
| ||||
| Hemoglobin, g/dL | 12.3±2.2 | 12.0±2.1 | 12.0±2.0 | 0.5 |
|
| ||||
| Sodium, mEq/L | 134.8±5.0 | 135.0±4.6 | 133.7±4.8 | 0.2 |
|
| ||||
| Right atrial pressure, mmHg | 11.9±6.1 | 12.5±6.9 | 12.4±7.0 | 0.6 |
|
| ||||
| Pulmonary capillary wedge | 25.2±10.0 | 25.5±9.2 | 23.6±9.9 | 0.5 |
|
| ||||
| pressure, mm Hg | ||||
|
| ||||
| Cardiac index, L/min/m2 | 1.8±0.5 | 1.8±0.5 | 2.2±2.6 | 0.06 |
|
| ||||
| Pulmonary vascular resistance, Woods units | 3.1±2.1 | 3.8±2.3 | 4.0±2.1 | 0.001 |
|
| ||||
| Class I PRA, % | 8.6±22.0 | 8.9±21.4 | 11.9±25.5 | 0.6 |
|
| ||||
| Class II PRA, % | 8.2±23.1 | 5.2±18.5 | 1.7±8.6 | 0.06 |
Values are mean ± standard deviation or N (%). ACHD, adult congenital heart disease; eGFR, estimated glomerular filtration rate; PRA, panel reactive antibody.
Association of insurance type with eligibility for HT/LVAD
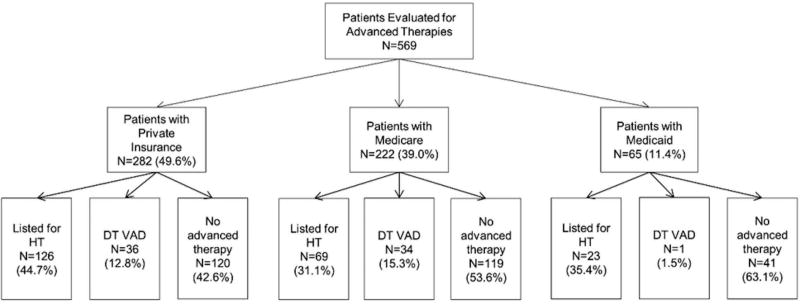
Overall, 218 (38.3%) patients were listed for HT, 71 (12.5%) received DT LVAD, and 280 (49.2%) were ineligible for advanced therapies. The outcome of the evaluation for HT/LVAD varied according to insurance type (Figure 1). On univariate analysis, patients with Medicaid insurance were least likely to be eligible for any advanced therapies (p=0.003). Patients with Medicaid insurance were least likely to receive DT LVAD (p=0.004), while patients with Medicare insurance were least likely to be eligible for HT (p=0.007). Other predictors of ineligibility for advanced therapies included female sex, BMI, serum albumin and inotropic support. Reasons for ineligibility for HT varied according to insurance type (Table 2). Medicaid patients had a higher mean number of reasons documented for ineligibility for HT. Patients with Medicare were more likely to be ineligible due to medical comorbidities, while patients with Medicaid were more likely to have inadequate social support. In order to examine whether insurance status is a significant variable in determining eligibility for DT LVAD, we compared the differences in comorbidities in those patients ineligible for HT in Table 3. Medicaid patients generally had a similar or more favorable medical profile, including younger age, better renal function, and lower BMI. When examining the reasons these patients were ineligible for HT, inadequate social support remained slightly more common in Medicaid patients who were considered ineligible for HT or DT LVAD. After excluding those patients who died during the evaluation process (N=26), patients with Medicare and Medicaid insurance were less likely to be eligible for HT in fully adjusted models. Moreover, patients with Medicaid insurance were 92% less likely than patients with private insurance to be eligible for DT LVAD, while there was no difference in eligibility for DT LVAD between patients with Medicare and private insurance (Table 4). Similarly, patients with Medicaid insurance were 89% less likely (OR 0.11, 95% CI 0.01 – 0.91; P=0.04) than patients with Medicare insurance to be eligible for DT LVAD.
Figure 1.
Outcomes of evaluation for advanced HF therapy by insurance type.
Table 2.
Primary reasons for ineligibility for HT in patients evaluated for advanced HF therapies according to insurance type.
| Private N=156 |
Medicare N=153 |
Medicaid N=42 |
P-value | |
|---|---|---|---|---|
| Medical contraindication | 58 (37.2) | 68 (44.4) | 10 (23.8) | 0.04 |
| Not inotrope dependent | 44 (28.2) | 25 (16.3) | 8 (19.1) | 0.03 |
| Inadequate social support | 12 (7.7) | 15 (9.8) | 9 (21.4) | 0.03 |
| Active substance abuse | 16 (10.3) | 11 (7.2) | 3 (7.1) | 0.6 |
| Death/rapid deterioration | 10 (6.4) | 10 (6.5) | 6 (14.3) | 0.2 |
| Age | 2 (1.3) | 13 (8.5) | 0 (0) | 0.003 |
| Inadequate financial resources | 4 (2.6) | 2 (1.3) | 3 (7.1) | 0.1 |
| Active non-compliance | 4 (2.6) | 1 (0.7) | 2 (4.8) | 0.2 |
| Other | 6 (3.9) | 8 (5.2) | 1 (2.4) | 0.7 |
| Total number of reasons | 1.3 ± 0.6 | 1.4 ± 0.8 | 1.7 ± 0.7 | 0.003 |
Values are N (%) or mean ± SD.
Table 3.
Comparison of comorbid conditions and reasons for HT ineligibility in those patients who received destination therapy left ventricular assist device (DT LVAD) compared to those considered ineligible for any advanced heart failure therapy.
| DT LVAD N=71 |
Ineligible for Advanced Therapies | P-value | |||
|---|---|---|---|---|---|
|
| |||||
| Private N=120 |
Medicare N=119 |
Medicaid N=41 |
|||
|
| |||||
| Age, years | 54.6±11.6 | 52.1±12.1 | 55.7±12.8 | 42.6±13.5‡ | <0.0001 |
|
| |||||
| Race | 0.06 | ||||
| • White | 36 (50.7) | 54 (50.7) | 51 (42.9) | 9 (22.0) | |
| • Black | 33 (46.5) | 57 (47.5) | 62 (52.1) | 30 (73.2)‡ | |
| • Other | 2 (2.8) | 9 (7.5) | 6 (5.0) | 2 (4.9) | |
|
| |||||
| Male | 34 (47.9) | 75 (62. 5)* | 77 (64.7)§ | 24 (58.5) | 0.1 |
|
| |||||
| Lung Disease | 12 (16.9) | 10 (8.3) | 23 (19.3) | 8 (19.5) | 0.08 |
|
| |||||
| LV thrombus | 7 (9.9) | 12 (10.0) | 14 (11.8) | 5 (12.2) | 0.9 |
|
| |||||
| Inotrope Dependent | 60 (84.5) | 44 (36.7)* | 64 (53.8)§ | 22 (53.7)‡ | <0.0001 |
|
| |||||
| BMI, kg/m2 | 31.6±8.1 | 30.1±7.6 | 29.6±7.8 | 27.1±6.7‡ | 0.03 |
|
| |||||
| Albumin, g/dL | 3.4±0.5 | 3.5±0.6 | 3.4±0.5 | 3.4±0.7 | 0.4 |
|
| |||||
| Total bilirubin, mg/dL | 1.4±1.1 | 1.5±1.3 | 1.6±1.1 | 1.9±1.5 | 0.2 |
|
| |||||
| Creatinine, mg/dL | 1.4±0.5 | 1.5±0.7 | 1.6±0.9 | 1.3±0.3 | 0.08 |
|
| |||||
| eGFR, ml/min/1.73 m2 | 53.6±20.0 | 57.7±25.0 | 56.1±25.0 | 69.4±23.4‡ | 0.007 |
|
| |||||
| Hemoglobin, g/dL | 12.2±2.1 | 11.8±2.4 | 11.9±2.1 | 11.9±2.2 | 0.7 |
|
| |||||
| CKD stage | 0.002 | ||||
| • 1–2 | 23 (32.4) | 52 (43.3) | 49 (41.2) | 28 (68.3)‡ | |
| • 3 | 42 (59.2) | 50 (41.7) | 55 (46.2) | 13 (31.7)‡ | |
| • 4–5 | 6 (8.5) | 18 (15.0) | 15 (12.6) | 0 (0) | |
|
| |||||
| Sodium, mEq/L | 134.1±4.2 | 135.4±4.9 | 135.0±5.0 | 133.7±4.8 | 0.1 |
|
| |||||
| RAP, mmHg | 11.9±6.6 | 11.9±6.2 | 11.9±7.2 | 13.3±7.4 | 0.8 |
|
| |||||
| PCWP, mm Hg | 25.6±9.8 | 23.4±10.3 | 25.2±9.8 | 24.3±10.2 | 0.5 |
|
| |||||
| CI, L/min/m2 | 1.7±0.4 | 1.9±0.6 | 1.9±0.5 | 2.4±3.3‡ | 0.04 |
|
| |||||
| Active non-compliance | 2 (2.8) | 2 (1.7) | 1 (0.8) | 2 (4.9) | 0.3 |
|
| |||||
| Death/rapid deterioration | 2 (2.8) | 9 (7.5) | 9 (7.6) | 6 (14.6)‡ | 0.2 |
|
| |||||
| Inadequate financial resources | 0 (0) | 4 (3.3) | 2 (1.7) | 3 (7.3)‡ | 0.09 |
|
| |||||
| Inadequate social support | 1 (1.4) | 11 (9.2)* | 15 (12.6)§ | 9 (22.0)‡ | 0.003 |
|
| |||||
| Active substance abuse | 7 (9.9) | 11 (9.2) | 9 (7.6) | 3 (7.3) | 0.9 |
BMI, body mass index; CI, cardiac index; CKD, chronic kidney disease; DT, destination therapy; eGFR, estimated glomerular filtration rate; LV, left ventricle; RA, right atrial pressure; PCWP, pulmonary capillary wedge pressure.
P<0.05 between DT LVAD vs private
P<0.05, DT vs medicare
P<0.05, DT vs Medicaid
Table 4.
Multivariate logistic regression to determine odds of being eligible for heart transplant or destination therapy LVAD. Model to determine odds of being eligible for heart transplant was run in the entire cohort. Model to determine odds of being eligible for destination therapy LVAD was only run in the patient determined to be ineligible for heart transplant. Models are adjusted for age, gender, race, heart failure etiology, body mass index, chronic kidney disease, albumin, and use of inotropes at discharge.
| Heart Transplant N=569 |
Destination Therapy LVAD N=351 |
|||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
|
| ||||
| Insurance type | ||||
| • Private | Reference | Reference | Reference | Reference |
| • Medicare | 0.58 (0.37–0.89) | 0.01 | 0.72 (0.38–1.35) | 0.3 |
| • Medicaid | 0.50 (0.25–0.99) | 0.04 | 0.08 (0.01–0.66) | 0.02 |
Association of insurance type with survival
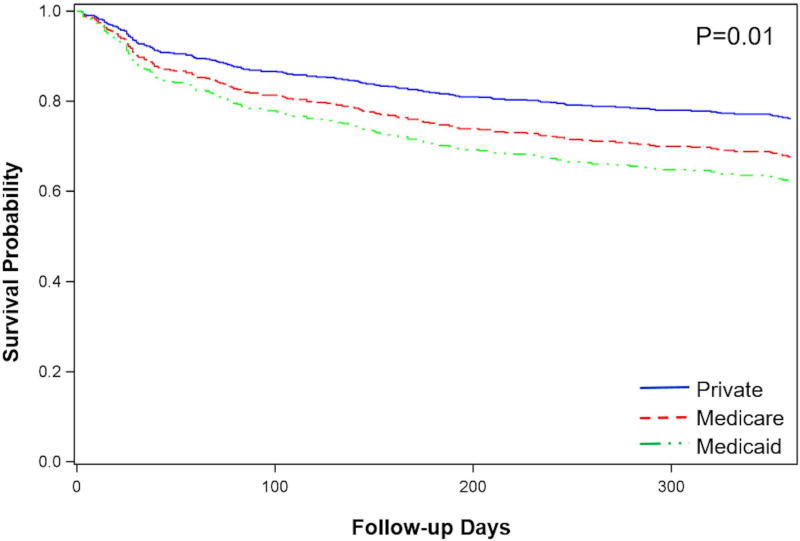
Overall, 336 (71.1%) patients evaluated for advanced HF therapies survived to one year after evaluation. Risk-adjusted survival differed by insurance type (Figure 2). After adjustment for covariates, Medicaid insurance was a significant predictor of death at one year (HR=2.15, 95% CI 1.31–3.52; p=0.002). Other predictors of death at one year included age (adjusted HR=1.02, 95% CI 1.01–1.04; p=0.01), CKD stage 3 (adjusted HR=1.48, 95% CI 1.03–2.14; p=0.04) or stage 4/5 (adjusted HR=2.02, 95% CI 1.20–3.38; p=0.008), dependence on continuous inotropes (adjusted HR=2.52, 95% CI 1.67–3.80; p<0.0001) and serum albumin (adjusted HR=0.67, 95% CI 0.50–0.88; p=0.005).
Figure 2.
Survival after initial evaluation for advanced therapies according to insurance type.
Probability of survival in patients who were eligible for HT
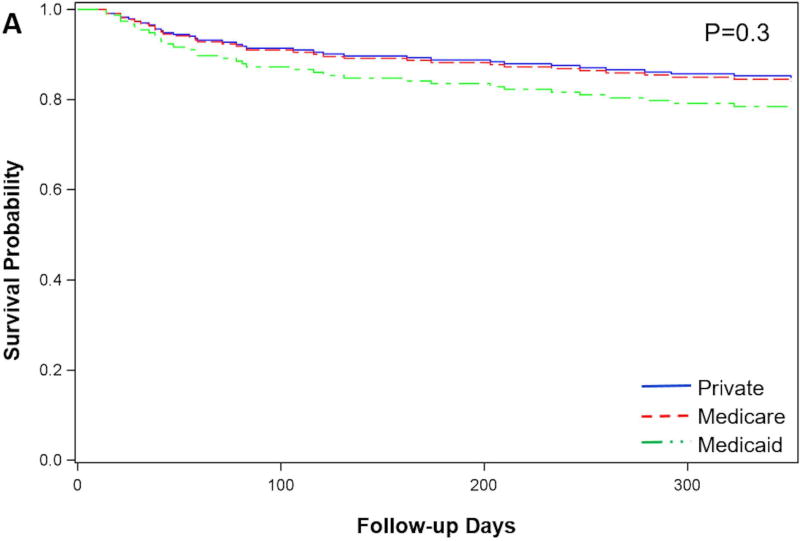
Overall survival at one year was higher for patients listed for HT as compared to patients not listed (83.8% vs 62.6%, P<0.0001). Among patients listed for HT, there was no difference in survival by insurance type (Figure 3A). Among patients listed for HT, there was no difference in the time from evaluation to listing for heart transplant (private 107.0 ± 196.7 vs. Medicare 100.4 ± 117.2 vs. Medicaid 154.3 ± 202.3 days; P=0.4), or in the proportion of patients initially listed as status 1A (private 40.8% vs. Medicare 39.4% vs. Medicaid 60.9%; P=0.1). LVAD as BTT was required for 77 (35.3%), with no difference by insurance type (private 40.5% vs. Medicare 31.4% vs. Medicaid 17.4%; P=0.2). Even after accounting for the competing event of transplant, the cumulative incidence of mortality on the wait list at one year did not differ significantly based on insurance type (private=15.6% vs. Medicare 14.7% vs. Medicaid 17.4%; P=0.9).
Figure 3.
Survival after initial evaluation for advanced therapies in patients not listed for heart transplant (3A) and patients listed for heart transplant (3B) according to insurance type.
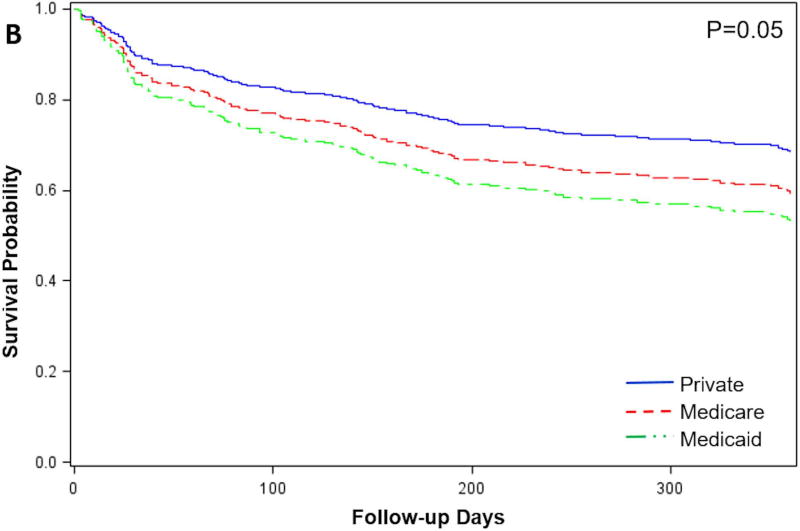
Probability of survival in patients who were not eligible for HT
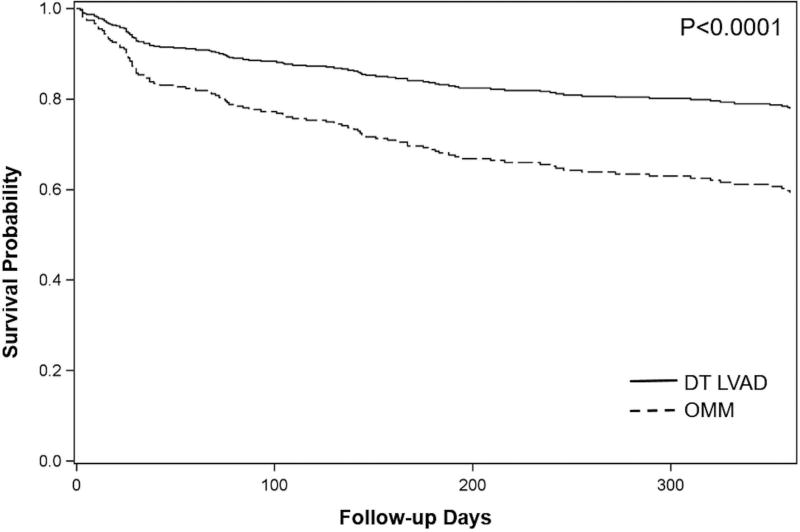
Among patients not listed for HT, survival differed according to insurance type (Figure 3B). Compared to patients with private insurance, patients with Medicaid had an increased risk of death (adjusted HR=2.04, 95% CI 1.14–3.67; p=0.01) while there was no increased risk in patients with Medicare insurance (adjusted HR=1.16, 95% CI 0.78–1.74; P=0.5). We further examined survival in patients who received DT LVAD vs. those who were ineligible for any advanced therapies and continued with optimal medical management (OMM). Survival was higher for patients who received DT LVAD vs. OMM (77.8% vs. 59.2%; P<0.0001) (Figure 4). Presumably, Medicaid patients could benefit from the 18.6% survival advantage afforded to DT LVAD recipients if their insurance benefits afforded full access to this therapy.
Figure 4.
Risk-adjusted survival one year after initial evaluation for advanced therapies in patients with Medicare or Private insurance not listed for HT, according to therapy. DT LVAD, destination therapy LVAD; OMM, optimal medical management.
DISCUSSION
In this single center retrospective cohort analysis, we found that 1) patients with Medicare and Medicaid insurance were less likely to be eligible for HT than patients with private insurance, but there was no difference in survival among patients based on insurance type once they were listed for HT, 2) patients with Medicaid insurance were significantly less likely than patients with private or Medicare insurance to be eligible for DT LVAD therapy, and 3) Medicaid insurance was a predictor of lower survival among patients not listed for HT despite fewer medical comorbidities. Since Medicaid patients generally had a more favorable medical profile, and were only slightly less likely to have inadequate social support than patients with private or Medicare insurance who received DT LVAD, we feel our data are highly suggestive that the Medicaid patients were 89–92% less likely to receive DT LVAD due to lack of insurance coverage for this therapy. Our data confirm the importance of adequate insurance coverage on access to lifesaving medical therapies. Since charity care services at our hospital pay for HT for patients with Medicaid insurance, their access to this therapy bypasses restrictions based on insurance as long as they meet other criteria. Moreover, if patients are eligible for HT, insurance type does not impact survival, presumably due to the comprehensive care offered once a patient is deemed eligible for the therapy. However, since charity care services have previously been unavailable for DT LVAD, Medicaid patients are less likely to be eligible for this treatment, with a profound negative impact on survival.
Previous studies have suggested disparities in access to heart and other solid organ transplants based on insurance type. In a retrospective review of United Network for Organ Sharing (UNOS) data, DuBay et al. found that patients with Medicaid received fewer HT than expected and were listed with more severe organ failure, suggesting listing at a later stage of illness.13 Studies of other solid organ transplants have shown clear differences in access by insurance type. Patients with public insurance are less likely to be evaluated or listed for kidney and liver transplant17,18,19 Although a similar proportion of patients with Medicaid and Medicare insurance were listed for HT in our cohort, the proportion was less than that of patients with private insurance, and the reasons patients were ineligible differed according to insurance type.
Patients with Medicaid may be less likely to be eligible for advanced HF therapies due to concern about psychosocial and financial risk factors which could negatively impact outcomes. In our study, patients with Medicaid were more likely to ineligible due to inadequate social support. Since Medicaid patients are often younger in age, a stable “primary social support person” for HT may be more difficult to obtain for a variety of reasons, including young spouses or other family members who work full time, minor children who are unable to serve as primary support, or older parents who may also have chronic health issues. A recent meta-analysis of 32 studies with 9,102 subjects suggests that social support may be weakly and inconsistently associated with post-transplant medication adherence.20 Still, most transplant programs consider adequate social support systems to be a requirement for transplant candidacy. Although previous studies have also reported increased likelihood of non-adherence among Medicaid patients after HT11,21,22, concerns about non-adherence did not differ by insurance type. Moreover, inadequate financial resources was not a factor determining differences in eligibility for HT by insurance type in our cohort.
We found that Medicaid insurance was associated with an increased risk of death among patients who were ineligible for advanced HF therapies. Our findings are consistent with a previous analysis by Foraker et al. demonstrating increased risk of death in Medicaid patients after index HF hospitalizations.23 Since patients with Medicaid had fewer medical comorbidities that might increase mortality, we suspect that the differential access to life-saving advanced HF therapies contributed to the survival difference observed in our cohort.1, 2,4 Although Medicaid insurance itself limits access to many medical therapies, Medicaid insurance is likely a proxy for other socioeconomic disadvantage that might further influence clinical outcomes, including limited access to regular health care, lower individual income, and/or lower health literacy.
Among patients who were listed for HT, we did not find a difference in survival by insurance type. Previous studies investigating patient outcomes after HT according to insurance have found differing results. Retrospective studies of UNOS data have found that public insurance to be associated with decreased long-term survival after HT.10,12 Another recent study which stratified UNOS HT data by geographic region found that public insurance was most prevalent in region 3, which includes Georgia.24 Although public insurance was not associated with an increased risk of death in region 3, it was associated with increased risk of death in regions 2, 10, and 11. Fewer data exist examining outcomes for patients with BTT LVAD. Smith et al. examined outcomes related to insurance type and socioeconomic status in 101 patients with the HeartMate II LVAD, including 41 (40.6%) who were BTT, and found no difference in the risk of death or hospitalization n in patients with Medicaid.25 We suspect this is related to rigorous patient selection to determine eligibility for advanced therapies, as well as stellar compliance on the part of patients who are awaiting HT, have been recently transplanted, or have undergone LVAD implantation.
Our findings are critical in the context of current and future changes facing the US healthcare system. After implementation of the Affordable Care Act (ACA), HT listings increased more in Medicaid expansion states, and more Medicaid patients were listed for transplant.26 Moreover, the ACA Medicaid expansion was associated with increased HT listings in African-American patients27, a group that is overrepresented in terms of HF severity at our center and nationwide, and more likely to be underinsured. Coverage for HT for our patients with Medicaid was provided through hospital charity care; however these patients do have prescription drug benefits through Medicaid that provide access to medication including maintenance immunosuppression. Georgia Medicaid provides limited reimbursement for LVAD implantation, however there is limited to no coverage for the cost of supplies for driveline dressing changes, such that the out-of-pocket cost to patients may be substantial. Since undertaking this scientific analysis, our hospital has agreed to provide charity care to help cover any unreimbursed costs of implantation as well as supplies for Medicaid patients requiring DT LVAD. Given the high cost of advanced heart failure therapies, adequate insurance coverage is critical to maintain equitable access to specialized medical and surgical therapies. In 2009, the national average cost of the index hospitalization for LVAD was $208,522 and for HT was $168,576.28 Financial pressure on states may continue to place coverage for specialized medical services in jeopardy, as organ transplant is not included in the core medical services which must be covered by state Medicaid programs. For instance, in 2010 Arizona chose to eliminate Medicaid coverage for heart, lung, liver, bone marrow and pancreas transplants in the face of a state budget deficit.29 Although Arizona reinstituted these benefits after drawing national attention and criticism, transplant services remain at risk. Our findings confirm that Medicaid patients tend to be younger, with fewer medical comorbidities, and potentially have the highest likelihood for gaining life years from transplant.30 Thus, it is essential to understand and address any disparities created by insurance coverage in HT or LVAD recipients.
Our study has several limitations. Our results are from a single center in a state with unique coverage polices for HT and LVAD for patients with Medicaid insurance; thus the generalizability of our findings need to be interpreted with caution. Although analysis of larger national databases would be valuable to determine if this is a state-specific issue, vs a national trend, we are not aware of any large databases (i.e. Scientific Registry of Transplant Recipients, Interagency Registry for Mechanically Assisted Circulatory Support) that capture data on patients who are evaluated vs. those who actually receive advanced HF therapies. Similarly, it is possible that patients with Medicaid insurance and uninsured patients were more likely to have never been referred for a HT or LVAD evaluation, thus limiting our ability to capture the true denominator of eligible patients. Some patients evaluated for HT and LVAD at our center returned to physicians outside of our system for continued care, limiting access to survival and outcome data which may bias our results. Additionally, patients were stratified by insurance type at the time of evaluation, but data on any subsequent changes in insurance type was not captured. However, any patients with a change in their status (medical, insurance, social support, etc) would have been re-evaluated, and all repeat evaluations recorded in the medical record were reviewed. We additionally acknowledge that some covariates which may influence patient outcomes (including individual income, education, and medication co-pays) are not captured in our medical record and thus not included in this analysis. Finally, our analysis does not address differences which may exist in referral to evaluation for advanced therapies.
In summary, our analysis demonstrates limited eligibility for DT LVAD for patients with Medicaid insurance, as well as an increased risk of death among patients with Medicaid insurance who are not listed for HT. Given the large and expanding economic burden of HF, it is critical to examine patterns of health resource use, associated impact on clinical outcomes, and disparities in access to life-saving therapies. In order to determine whether increased access to advanced HF therapies is feasible for our state government, cost-effectiveness analyses should be performed, in addition to the design of patient-centered interventions that can be tested in future analyses to target access barriers specific to patients with Medicaid.
Acknowledgments
SOURCES OF FUNDING
The project was supported by funding from NIH/NIMHD U54 MD008173. AAM is also supported by funding from NIH/NHLBI K23 HL124287 and the Robert Wood Johnson Foundation (Harold Amos Medical Faculty Development Program).
Footnotes
DISCLOSURES
The authors have no conflicts of interest to disclose.
References
- 1.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-Term Use of a Left Ventricular Assist Device for End-Stage Heart Failure. New England Journal of Medicine. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 2.Rogers JG, Butler J, Lansman SL, et al. Chronic Mechanical Circulatory Support for Inotrope-Dependent Heart Failure Patients Who Are Not Transplant Candidates: Results of the INTrEPID Trial. Journal of the American College of Cardiology. 2007;50:741–7. doi: 10.1016/j.jacc.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson LW, Miller LW, Desvigne-Nickens P, et al. Left Ventricular Assist Device as Destination for Patients Undergoing Intravenous Inotropic Therapy. A Subset Analysis From REMATCH (Randomized Evaluation of Mechanical Assistance in Treatment of Chronic Heart Failure) 2004;110:975–81. doi: 10.1161/01.CIR.0000139862.48167.23. [DOI] [PubMed] [Google Scholar]
- 4.Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report--2015; Focus Theme: Early Graft Failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1244–54. doi: 10.1016/j.healun.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Colvin M, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 Annual Data Report: Heart. American Journal of Transplantation. 2017;17:286–356. doi: 10.1111/ajt.14128. [DOI] [PubMed] [Google Scholar]
- 6.Boothroyd LJ, Lambert LJ, Sas G, et al. Should Eligibility for Heart Transplantation Be a Requirement for Left Ventricular Assist Device Use? Recommendations Based on a Systematic Review. Canadian Journal of Cardiology. 2013;29:1712–20. doi: 10.1016/j.cjca.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: A 10,000-patient database. The Journal of Heart and Lung Transplantation. 2014;33:555–64. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. The Journal of Heart and Lung Transplantation. 35:1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: Executive summary. The Journal of Heart and Lung Transplantation. 32:157–87. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Allen JG, Weiss ES, Arnaoutakis GJ, et al. Insurance and education predict long-term survival after orthotopic heart transplantation in the United States. The Journal of Heart and Lung Transplantation. 2012;31:52–60. doi: 10.1016/j.healun.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Oliva M, Singh TP, Gauvreau K, VanderPluym CJ, Bastardi HJ, Almond CS. Impact of medication non-adherence on survival after pediatric heart transplantation in the USA. The Journal of Heart and Lung Transplantation. 2013;32:881–8. doi: 10.1016/j.healun.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Tumin D, Foraker RE, Smith S, Tobias JD, Hayes D., Jr Health Insurance Trajectories and Long-Term Survival After Heart Transplantation. Circulation Cardiovascular quality and outcomes. 2016;9:576–84. doi: 10.1161/CIRCOUTCOMES.116.003067. [DOI] [PubMed] [Google Scholar]
- 13.DuBay DA, MacLennan PA, Reed RD, et al. Insurance Type and Solid Organ Transplantation Outcomes: A Historical Perspective on How Medicaid Expansion Might Impact Transplantation Outcomes. Journal of the American College of Surgeons. 2016;223:611–20.e4. doi: 10.1016/j.jamcollsurg.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thibodeau JT, Rao MP, Gupta C, et al. Health Insurance as a Requirement to Undergo Cardiac Transplantation: A National Survey of Transplant Program Practices. Transplantation Proceedings. 2012;45:360–3. doi: 10.1016/j.transproceed.2012.05.074. [DOI] [PubMed] [Google Scholar]
- 15.Herring A, Woolhandler S, Himmelstein D. Insurance status of U.S. organ donors and transplant recipients: the uninsured give, but rarely receive. International journal of health services: planning, administration, evaluation. 2008;38:641–52. doi: 10.2190/HS.38.4.d. [DOI] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 17.Johansen KL, Zhang R, Huang Y, Patzer RE, Kutner NG. Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clinical journal of the American Society of Nephrology : CJASN. 2012;7:1490–7. doi: 10.2215/CJN.13151211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patzer RE, Plantinga LC, Paul S, et al. Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in georgia. JAMA. 2015;314:582–94. doi: 10.1001/jama.2015.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryce CL, Angus DC, Arnold RM, et al. Sociodemographic differences in early access to liver transplantation services. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:2092–101. doi: 10.1111/j.1600-6143.2009.02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladin K, Daniels A, Osani M, Bannuru RR. Is social support associated with post-transplant medication adherence and outcomes? A systematic review and meta-analysis. Transplantation Reviews. 2017 doi: 10.1016/j.trre.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dew MA, DiMartini AF, De Vito Dabbs A, et al. Adherence to the Medical Regimen During the First Two Years After Lung Transplantation. Transplantation. 2008;85:193–202. doi: 10.1097/TP.0b013e318160135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumin D, McConnell PI, Galantowicz M, Tobias JD, Hayes D., Jr Reported Nonadherence to Immunosuppressive Medication in Young Adults After Heart Transplantation: A Retrospective Analysis of a National Registry. Transplantation. 2017;101:421–9. doi: 10.1097/TP.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 23.Foraker RE, Rose KM, Suchindran CM, Chang PP, McNeill AM, Rosamond WD. Socioeconomic Status, Medicaid Coverage, Clinical Comorbidity, and Rehospitalization or Death After an Incident Heart Failure Hospitalization. Atherosclerosis Risk in Communities Cohort (1987 to 2004) 2011;4:308–16. doi: 10.1161/CIRCHEARTFAILURE.110.959031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foraker RE, Tumin D, Smith S, Tobias JD, Hayes D., Jr Insurance status by region at the time of heart transplantation: Implications for survival. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2016;35:1480–6. doi: 10.1016/j.healun.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Smith SA, Hasan AK, Binkley PF, Foraker RE. The impact of insurance and socioeconomic status on outcomes for patients with left ventricular assist devices. Journal of Surgical Research. 2014;191:302–8. doi: 10.1016/j.jss.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira GH, Al-Kindi SG, Simon DI. Implementation of the Affordable Care Act and Solid-Organ Transplantation Listings in the United States. JAMA cardiology. 2016;1:737–8. doi: 10.1001/jamacardio.2016.2067. [DOI] [PubMed] [Google Scholar]
- 27.Breathett K, Allen LA, Helmkamp L, et al. The Affordable Care Act Medicaid Expansion Correlated With Increased Heart Transplant Listings in African-Americans But Not Hispanics or Caucasians. JACC: Heart Failure. 2017;5:136–47. doi: 10.1016/j.jchf.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulloy DP, Bhamidipati CM, Stone ML, Ailawadi G, Kron IL, Kern JA. Orthotopic heart transplant versus left ventricular assist device: a national comparison of cost and survival. The Journal of thoracic and cardiovascular surgery. 2013;145:566–73. doi: 10.1016/j.jtcvs.2012.10.034. discussion 73–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pondrom S. The AJT Report: News and issues that affect organ and tissue transplantation. Death by Budget Cuts? Arizona targets transplantation for Medicaid reductions. American Journal of Transplantation. 2011;11:641–2. doi: 10.1111/j.1600-6143.2011.03520.x. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe R, McCullough K, Schaubel D, et al. Calculating Life Years from Transplant (LYFT): Methods for Kidney and Kidney-Pancreas Candidates. American Journal of Transplantation. 2008;8:997–1011. doi: 10.1111/j.1600-6143.2008.02177.x. [DOI] [PubMed] [Google Scholar]