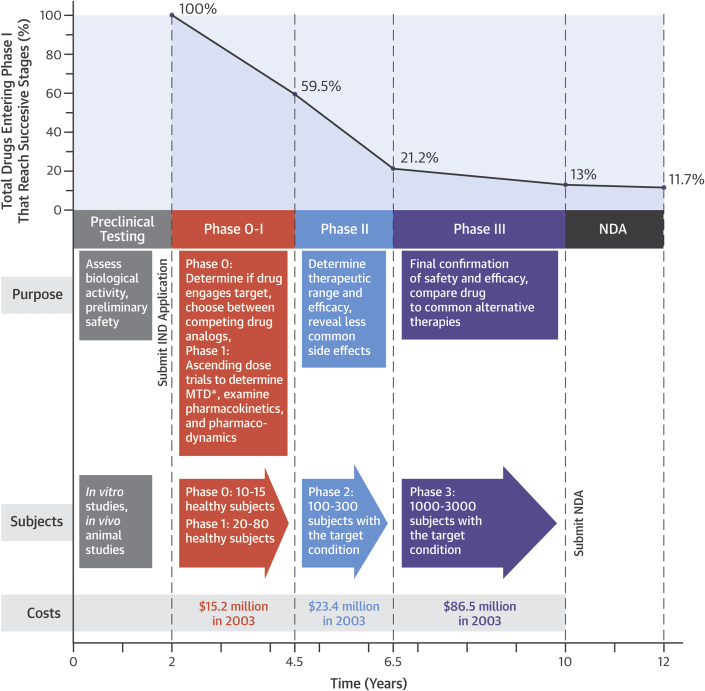
Figure 1.
Time, Money, and Success: Stages in Drug Development
The highest failure rates occurs in Phase II testing, which is the first stage in which doses of drug in humans are escalated to reach levels expected to be clinically active (i.e., the first doses at which efficacy may fail and less common side effects appear). Cumulative probability of a drug reaching U.S. Food and Drug Administration approval declines with each stage. The overall probability of a drug passing all stages is approximately 11% as of 2014 (12).