Fig. 3.
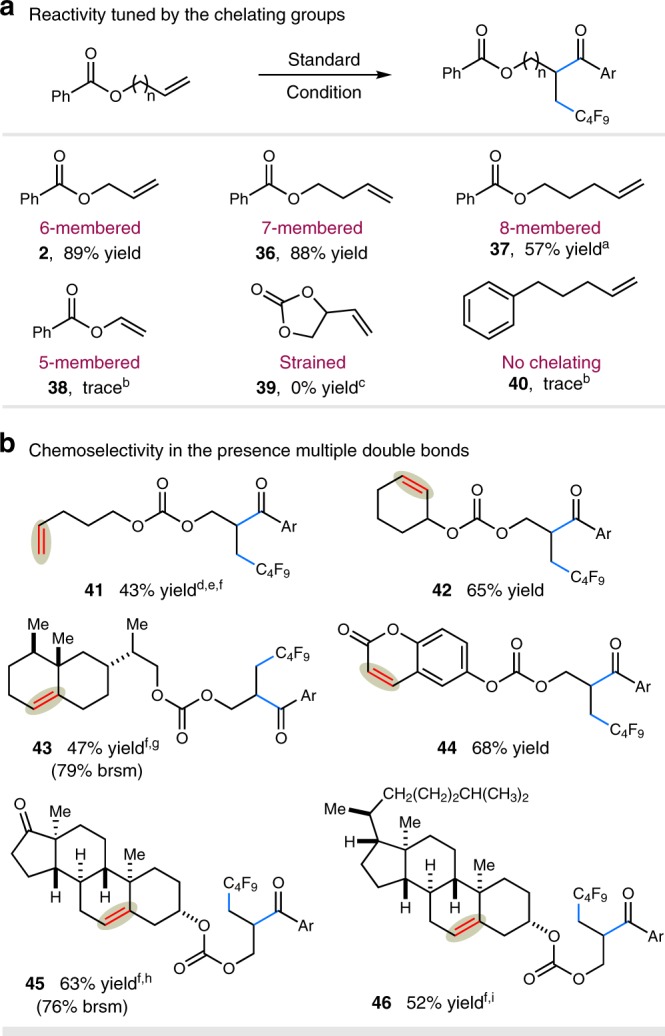
Chemoselectivity guided by pendant chelating groups. a Reactivity tuned by the chelating group. b Chemoselectivity in the presence of multiple double bonds. Reaction conditions: NiCl2•glyme (10 mol%), dtbbpy (20 mol%), alkene (1.0 equiv.), C4F9I (1.0 equiv.), acyl chloride (1.5 equiv.), Mn (3.0 equiv.), CH3CN [0.1 M], 25 oC, 20 h, see Supplementary Methods. All cited yields are isolated yields. Ar = 4-tert-butylphenyl. a36% of C4F9-alkene byproduct isolated. bAlkene consumed. cAlkene remained. dPerformed with 2 equiv. of alkene. e140% of alkene recovered. fPerformed in 4:1 CH3CN/DME [0.05 M]. g41% of alkene recovered. h17% of alkene recovered. i48 h
