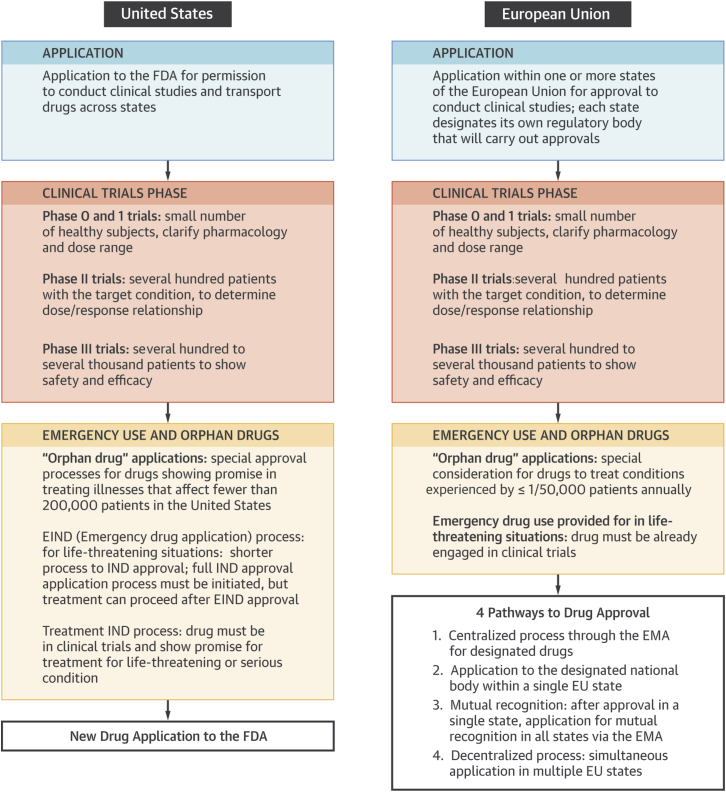
Figure 1.
Comparison of Drug Approval Processes in the United States and EU
After clinical trials, FDA drug approvals follow a centralized path, whereas European approval can occur through 4 different paths, depending on the nature of the drug and the preference of the manufacturer. EIND = emergency investigational new drug; EMA = European Medicines Agency; EU = European Union; FDA = Food and Drug Administration; IND = investigational new drug.