Figure 1.
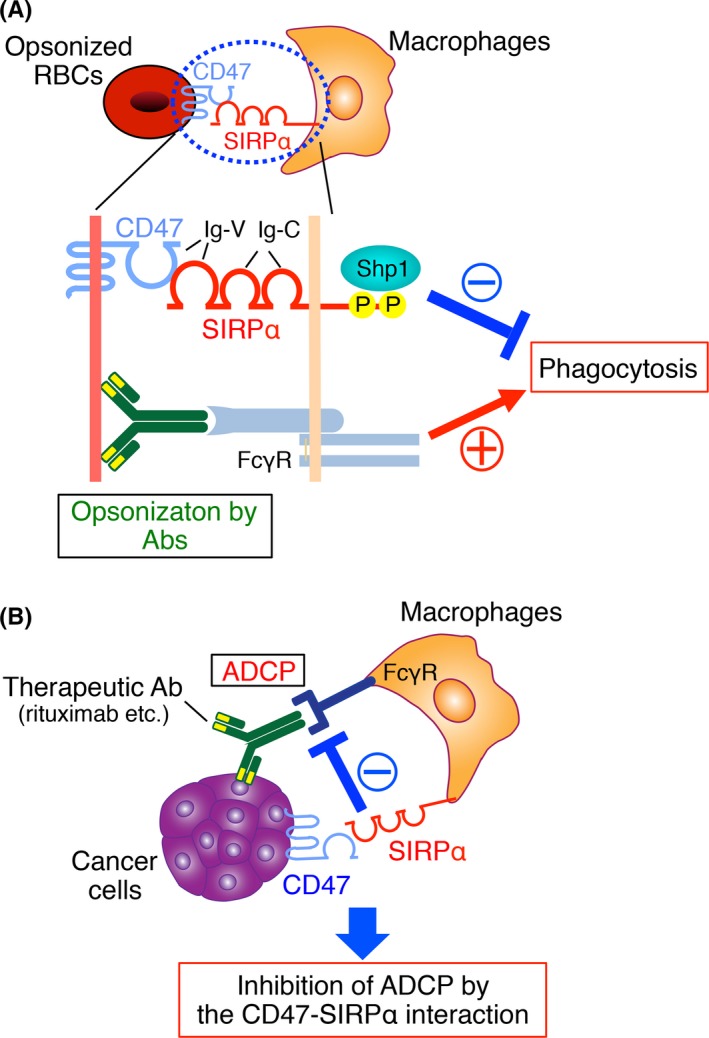
The CD47‐signal regulatory protein α (SIRPα) signaling system and its role in the regulation of phagocytosis by macrophages. A, SIRPα is a transmembrane protein that contains 3 Ig‐like domains (1 V‐like and 2 C1‐like Ig domains) in its NH 2‐terminal extracellular region and 2 key tyrosine phosphorylation sites in its COOH‐terminal cytoplasmic region. The tyrosine‐phosphorylated sites of SIRPα bind and thereby activate the protein tyrosine phosphatases Shp1 and Shp2. The SIRPα ligand CD47 is also a member of the Ig superfamily, with an Ig‐V‐like extracellular domain, 5 membrane‐spanning segments, and a short cytoplasmic tail. The Ig‐V‐like domain of CD47 interacts in trans with the NH 2‐terminal Ig‐V‐like domain of SIRPα and thereby triggers the tyrosine phosphorylation of the latter protein. Ligation of SIRPα on macrophages by CD47 on opsonized red blood cells (RBCs) thus promotes tyrosine phosphorylation of SIRPα and its subsequent association with Shp1, resulting in inhibition of RBCs phagocytosis by the macrophages elicited by the interaction of the Fc region of RBC‐bound Abs with the macrophage Fcγ receptor (FcγR). B, Interaction of CD47 on tumor cells with SIRPα on macrophages attenuates phagocytosis by the macrophages of the tumor cells triggered by opsonization with tumor antigen‐specific therapeutic Abs such as rituximab. ADCP, antibody‐dependent cellular phagocytosis
