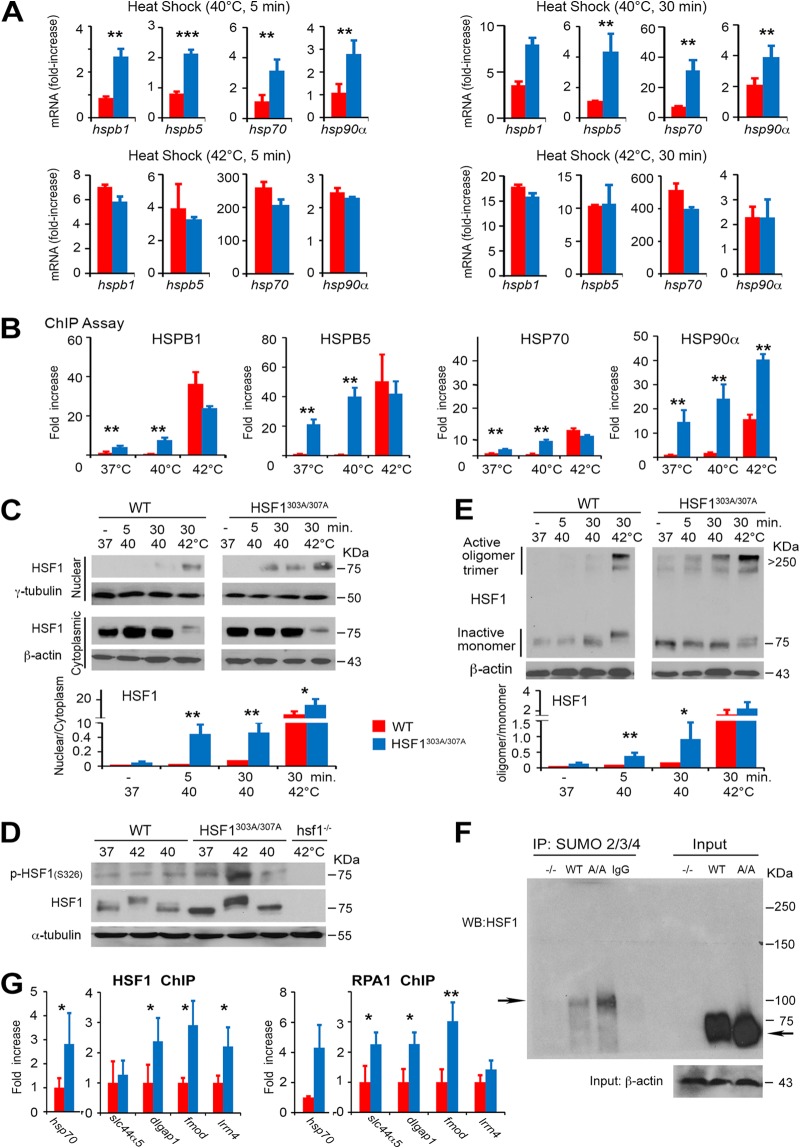
FIG 3.
Phosphorylation on S303/S307 defines the activation threshold of HSF1 to heat stress. (A) Heat stress-induced HSF1 target gene expression is enhanced upon mutation of S303/S307 phosphorylation. mRNA transcript levels of selected HSF1 target genes (hsp70, hspb1, hspb5, and hsp90α) in WT and HSF1303A/307A MEFs exposed to a different intensity of heat stress (40°C or 42°C for 5 or 30 min) and following recovery at 37°C for 1 h. Values are presented as relative mRNA expression compared to controls (WT and HSF1 mutant cells at 37°C) (n = 5 assays). (B) Increase in the DNA-binding capacity of HSF1 upon loss of S303/S307 phosphorylation. ChIP quantitative PCR (qPCR) assessing HSF1 occupancy at the HSE containing promoter regions of hspb1, hspb5, hsp70, or hsp90α in WT or HSF1303A/307A MEFs. Cells were either left untreated or exposed to heat shock (37°C, 40°C, or 42°C for 5 min). For quantification of HSF1 binding capacity, the values of qPCR normalized to the input values were expressed relative to WT control at 37°C, which was arbitrarily set at 1 (n = 4 assays). (C) Heat-induced HSF1 nuclear translocation increased following loss of phosphorylation on S303/S307. Immunoblot analysis of HSF1 in cytoplasmic and nuclear fractions prepared from WT and HSF1303A/307A MEFs and exposed to heat stress (37°C, 40°C, or 42°C for 5 or 30 min.). β-Actin and γ-tubulin were used as loading controls for cytoplasmic and nuclear fractions, respectively. The graph shows quantification of the HSF1 protein ratio in cytosolic and nuclear fractions normalized to the loading control level (right) (n = 3 analyses). (D) WT and HSF1303A/307A MEFs were exposed to heat shock (37°C, 40°C, or 42°C for 30 min), followed by recovery for 30 min at 37°C. Total cell lysates were analyzed by immunoblotting as indicated. α-Tubulin was used as a loading control. Lysates from hsf1−/− MEFs exposed to heat shock (42°C for 30 min) were used as a control for antibody specificity (n = 3 analyses). (E) High-molecular-weight oligomer of HSF1 formed during heat shock is stabilized by loss of S303/S307 phosphorylation. WT and HSF1303A/307A MEFs were exposed to heat shock (37°C, 40°C, or 42°C for 5 or 30 min) as indicated. Cell lysates prepared immediately after heat shock were subjected to cross-linking and blotted for HSF1. Ratios of active HSF1 (oligomers at 250 to 300 kDa) to inactive HSF1 monomer are shown (n = 3 analyses). (F) Representative endogenous analysis of SUMO-2/3/4-modified HSF1 in MEFs. Sumo-conjugated HSF1 was immunoprecipitated (IP) from MEF extracts from WT and mutant HSF1 (A/A) mice with an anti-SUMO-2/3/4 antibody and analyzed by Western blotting with antibody to HSF1. The arrows indicate high molecular mass of endogenous SUMO-modified HSF1 and unmodified native WT or mutant HSF1. β-Actin was used as a loading control. MEFs from whole-body HSF1 deletion mice (−/−) were included as a control (n = 2 analyses). (G) ChIP quantitative PCR assessing HSF1 (WT and HSF1303A/307A) and RPA1 binding at the putative HSE containing promoter region of hsp70, slc44a5, dlgap, fmod, and lrrn4 in MEFs of WT and HSF1 mutant mice. Analyses were performed using HSF1 or RPA1 antibody (n = 3 analyses). For all panels, bars are means ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Red, WT cells; blue, HSF1303A/307A cells.