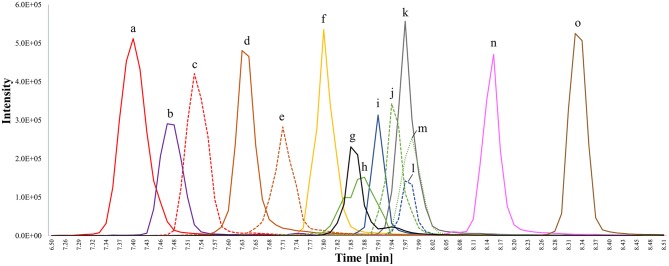
Figure 2.
Overlay of extracted ion chromatograms (EIC) for chromatographic separation of free sterol (FS) standards detected as [FS-H2O+H]+ on a C18 UPLC column applying a methanol/water gradient with 0.1% formic acid as additive; isomeric compounds have the same color and are distinguished by different line patterns. (a) Desmosterol (m/z 367; C27; Δ5,Δ24(25)); (b) ergosterol (m/z 379; C28; Δ5,Δ7,Δ22); (c) 7-dehydrocholesterol (m/z 367; C27; Δ5,Δ7); (d) 24-methylenecholesterol (m/z 381; C28; Δ5,Δ24(241)); (e) brassicasterol (m/z 381; C28; Δ5,Δ22); (f) cholesterol (m/z 369; C27, Δ5); (g) lanosterol (m/z 409; C30, Δ8); (h) Δ5-avenasterol extracted from oat (m/z 395; C29, Δ5,Δ24(241)); (i) coprostanol (m/z 371; C27, stanol with β-configuration of hydrogen at C5); (j) spinasterol (m/z 395; C19; Δ7,Δ22); (k) campesterol (m/z 383; C28; Δ5); (l) cholestanol (m/z 371; C27, stanol with α-configuration of hydrogen at C5); (m) stigmasterol (m/z 369; C29; Δ5,Δ22); (n) sitosterol (m/z 397; C29; Δ5), and (o) sitostanol (m/z 399; C29; fully saturated). Accurate m/z are listed in Table 1 and peaks were extracted from chromatograms with a mass window of 0.01 Da.