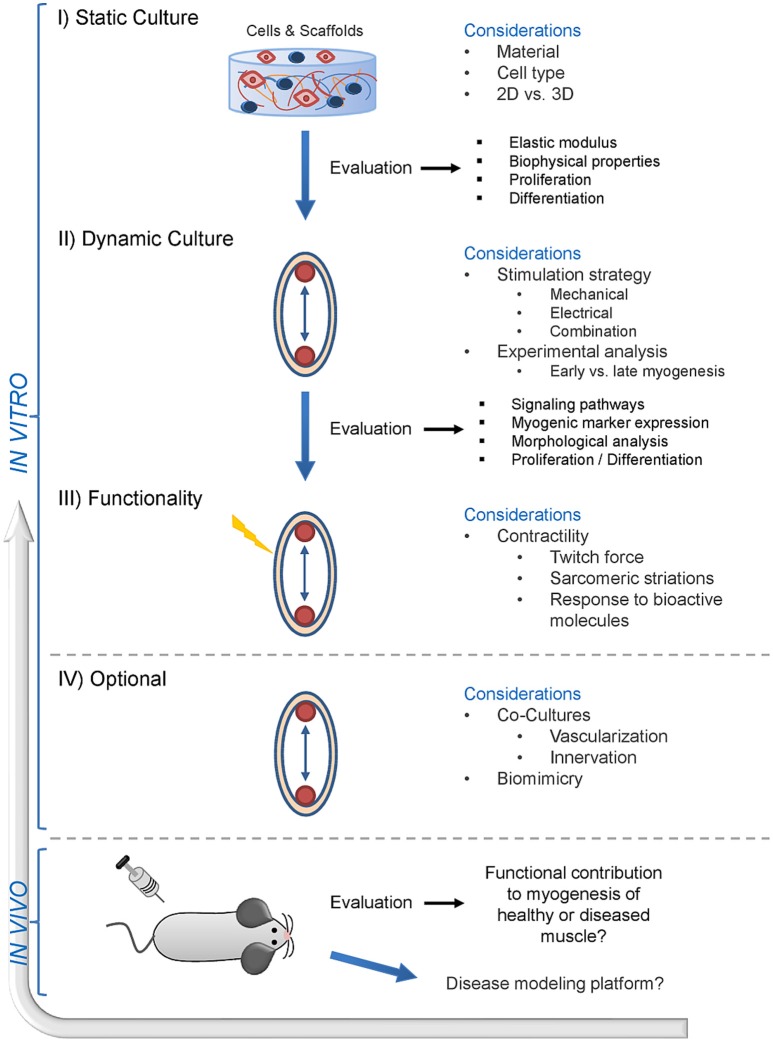
Figure 3.
Envisioned future of skeletal muscle tissue engineering—a suggested workflow. This schematic presents a skeletal muscle tissue engineering workflow including stage-specific experimental considerations. Initially, the compatibility of biomaterials with potent myogenic cells has to be evaluated. This first step also involves the decision whether the cells will be cultured and grown in a 2D (monolayer on a pliant matrix) or 3D (encapsulation into a pliant matrix) environment. This still represents a static cell culture, where only the first steps in the SMTE approach are addressed. Evaluation of the biophysical matrix properties, biocompatibility and effects of the biomaterial on cell proliferation/differentiation can be evaluated via this process. The second step involves dynamic culture of the evaluated biomaterial and cells, where the main consideration is which stimulation strategy will be implemented into the culture system—ranging from mechanical to electrical stimulation or a combination of both. The third step addresses the functional analysis of the engineered muscle construct via twitch force measurements. At this point, contractile muscle constructs can furthermore be tested for their response to drugs with known effects, which is a prerequisite for later application of engineered muscle tissue in drug screening studies. An ideal setup would involve co-cultures to engineer muscle tissue with built-in vascular and neuronal structures to further enhance muscle maturity and contractility. After successful in vitro evaluation, the final step is the translation into animal models to test for the contribution of the engineered muscle to myogenesis and regeneration in healthy and/or diseased muscle. Ultimately, the knowledge gained from in vivo experiments can also be transferred back to in vitro setups for the generation of disease models.