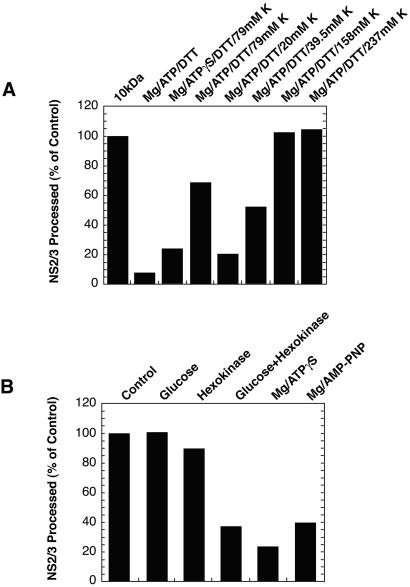
Figure 1.
HCV NS2/3 processing is supported by low molecular weight components of reticulocyte lysate. (A) NS2/3 protease was synthesized in vitro with 35S-methionine labeling, desalted, and recombined with selected low molecular weight components of RRL. Activity is expressed as a percent of the amount of NS2/3 cleavage achieved by recombination with the 10-kDa filtrate of RRL. Lysate containing translated, 35S-methionine-labeled NS2/3 (810–1615BK) was centrifuged through a spin column containing P-6 polyacrylamide gel (Bio-Rad; exclusion limit 6,000 Da) equilibrated in 20 mM Tris⋅HCl buffer, pH 7.5. The filtered lysate was diluted 10-fold into the column buffer or into buffer containing combinations of 1 mM ATP, 1.5 mM Mg(OAc)2, 2 mM DTT, and KOAc. In some samples, the amount of KOAc was varied (20–237 mM) or ATP-γ-S was substituted for ATP. Analyses were performed on SDS gels and quantified as described in Materials and Methods. (B) 35S-methionine-labeled NS2/3 (810–1615BK) was synthesized in RRL and 5-μl aliquots were directly combined with 1 μl of 500 mM glucose, 0.5 unit yeast hexokinase, or glucose plus hexokinase at the aforementioned concentrations, and incubated for 30 min at room temperature. Similarly, lysate containing NS2/3 was incubated with Mg/ATP-γ-S or Mg/adenosine 5′-[β,γ−imido]triphosphate, for final concentrations of the nucleoside analogs of 5 mM. The data shown are typical of additional experiments in which numerous other solutes were tested either alone or in combination.