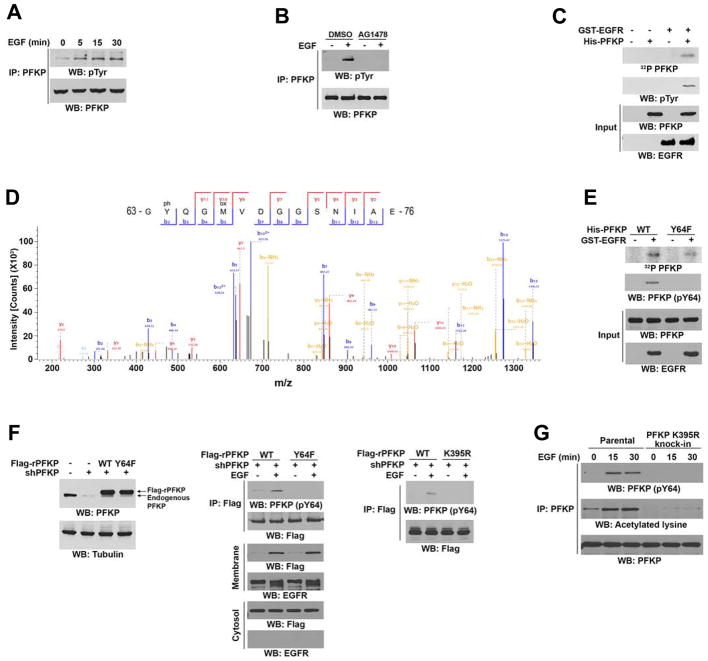
Figure 2. EGFR Phosphorylates PFKP at Y64.
Immunoblotting analyses were performed with the indicated antibodies (A–C, E–G).
(A) Serum-starved U251 cells were treated with or without EGF (100 ng/ml) for the indicated periods of time. Endogenous PFKP was immunoprecipitated.
(B) Serum-starved U251 cells were treated with or without AG1478 (1 μM) for 60 min before stimulation with or without EGF (100 ng/ml) for 15 min. Endogenous PFKP was immunoprecipitated.
(C, D) In vitro kinase assays were performed with purified bacterially expressed His-PFKP with or without GST-EGFR in the presence of Flag-KAT5 and Ac-CoA (C). Mass spectrometry analyses of a tryptic fragment of PFKP at a mass-to-charge ratio (m/z) of 747.28 (mass error, −0.05 ppm) matched the +2 charged peptide 63-YQGMVDGGSNIA-76, suggesting that Y64 was phosphorylated. The Mascot score was 204.18 (D).
(E) In vitro kinase assays were performed with purified bacterially expressed WT His-PFKP or His-PFKP Y64F mutant with or without GST-EGFR in the presence of Flag-KAT5 and Ac-CoA.
(F) U251 cells with or without PFKP depletion and reconstituted expression of WT Flag-rPFKP, Flag-rPFKP Y64F (left panel and middle panel), or Flag-rPFKP K395R (right panel) were treated with or without EGF (100 ng/ml) for 15 min. Cell fractionation and immunoprecipitation analyses were performed.
(G) Parental and the U251 cells with knock-in of PFKP K395R were treated with or without EGF (100 ng/ml) for the indicated time periods. MG132 (10 μM) was added to the cells 6 h before harvesting to eliminate the potential effect of proteasomal degradation of PFKP. Endogenous PFKP was immunoprecipitated.
See also supplementary Table S2.