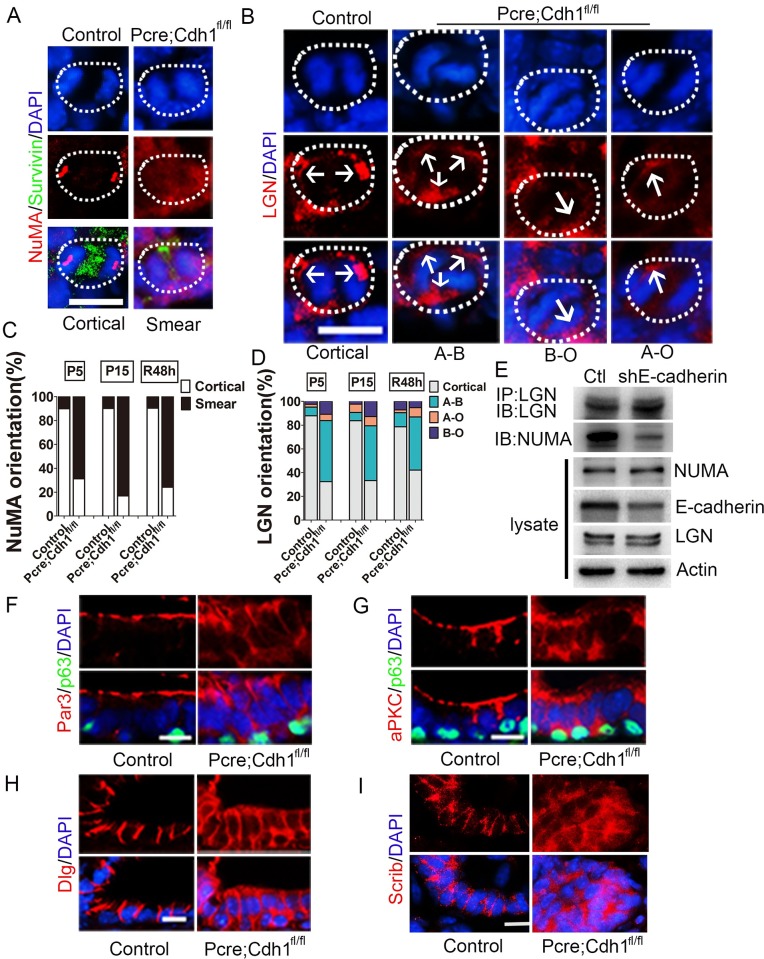
Fig 6. Ablation of E-cadherin disrupts the distribution of key spindle positioning and cell polarity proteins of prostate luminal cells.
(A) Immunofluorescent staining of NUMA shows that E-cadherin depletion in the mouse prostate diminishes NUMA cortical enrichment. (B) LGN can be detected all around the cell cortex of dividing E-cadherin knockout prostate epithelial cells, in comparison to its exclusively lateral distribution in wild-type controls. A-B, diffused LGN position; B-O, basal surface enriched LGN position; A-O, apical surface enriched LGN position. (C) Quantification of the NUMA localization pattern in dividing luminal cells during prostate development and regeneration. (D) Quantification of ratios of each type of LGN distributions in dividing luminal cells during prostate development and regeneration. (E) The formation of LGN/NUMA protein complex is markedly suppressed due to E-cadherin knockdown in vitro. (F-G) Wild-type prostate luminal cells exhibit apical distribution of polarity proteins PAR3 (F) and aPKC (G), whereas E-cadherin deleted luminal cells display a diffused expression pattern of those polarity proteins. (H-I) Immunostaining of basolateral polarity proteins, DLG (H) and SCRIB (I) shows that the basolateral polarity of luminal cells is dispersed by E-cadherin ablation. All sections were collected from P15 prostate tissues and counterstained by 4’,6-diamidino-2-phenylindole(DAPI)(blue) (Scale bars are10μm).