Abstract
Botulinum toxin type A (BoNT/A) has been used therapeutically for various conditions including dystonia, cerebral palsy, wrinkle, hyperhidrosis and pain control. The substantia gelatinosa (SG) neurons of the trigeminal subnucleus caudalis (Vc) receive orofacial nociceptive information from primary afferents and transmit the information to higher brain center. Although many studies have shown the analgesic effects of BoNT/A, the effects of BoNT/A at the central nervous system and the action mechanism are not well understood. Therefore, the effects of BoNT/A on the spontaneous postsynaptic currents (sPSCs) in the SG neurons were investigated. In whole cell voltage clamp mode, the frequency of sPSCs was increased in 18 (37.5%) neurons, decreased in 5 (10.4%) neurons and not affected in 25 (52.1%) of 48 neurons tested by BoNT/A (3 nM). Similar proportions of frequency variation of sPSCs were observed in 1 and 10 nM BoNT/A and no significant differences were observed in the relative mean frequencies of sPSCs among 1–10 nM BoNT/A. BoNT/A-induced frequency increase of sPSCs was not affected by pretreated tetrodotoxin (0.5 µM). In addition, the frequency of sIPSCs in the presence of CNQX (10 µM) and AP5 (20 µM) was increased in 10 (53%) neurons, decreased in 1 (5%) neuron and not affected in 8 (42%) of 19 neurons tested by BoNT/A (3 nM). These results demonstrate that BoNT/A increases the frequency of sIPSCs on SG neurons of the Vc at least partly and can provide an evidence for rapid action of BoNT/A at the central nervous system.
Keywords: Botulinum toxin type A, Pain, Spontaneous postsynaptic current, Substantia gelatinosa, Whole-cell recoding
INTRODUCTION
Botulinum neurotoxin (BoNT) causes muscular contraction and nerve paralysis by blocking acetylcholine (Ach) release at the neuromuscular junction [1]. BoNT has seven different serotypes (A-G) based on the antigenic specificity and reductions in muscle fiber activity [2,3]. BoNT binds to synaptic terminals and following endocytosis into cytoplasm cleaves soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins, vesicle-associated membrane protein (known as synaptobrevin), synaptosomal-associated protein 25 (SNAP-25) and syntaxin [4]. The cleavage of these proteins interferes with SNARE complex assembly, and subsequently inhibits exocytosis of synaptic vesicles containing neurotransmitters [5,6].
In the 1980s, BoNT/A was used as an alternative to conventional surgery in human strabismus and was extended to various neurological disorders related to spasmodic muscle contractions [7]. Furthermore, it has been reported that BoNT/A might be useful for the control of commonly occurring pain syndromes, such as trigeminal neuropathy, myofascial pain, migraine and lower back pain [8]. Currently, BoNT/A has been approved by the FDA (Food and Drug Administration) for treatment of blepharospasm, strabismus, focal dystonia, hemifacial spasm, spasticity, chronic migraine, hyperhidrosis and wrinkles [9,10].
In animal studies, peripheral BoNT/A application into the paw pad reduced or completely abolished sensitivities to thermal or mechanical stimuli using capsaicin or carrageenan in rat [11], and suppressed formalin-induced nociceptive behaviors [12,13]. Moreover, intrathecal injection of BoNT/A reduced formalin-induced nociceptive responses in mice [13,14] and significantly reduced bilateral pain induced by injection of acidic saline in rats [15].
The trigeminal subnucleus caudalis (Vc) is one of the principal relay sites for orofacial nociceptive information [16]. The substantia gelatinosa (SG; lamina II layer) receives Ad- and C-primary afferent inputs and plays a key role in the processing and transmission of nociceptive information [17,18].
Although a number of studies have shown analgesic effects of BoNT/A in neuropathic and/or inflammatory pain, the direct effects of BoNT/A on the central nervous system (CNS) and its action mechanism are not well elucidated. Therefore, in this study, the effects of BoNT/A on the spontaneous postsynaptic currents (sPSCs) in the SG neurons of the Vc were investigated using patch clamp technique.
METHODS
Animals
All experiments were approved by the Chonbuk National University Animal Welfare and Ethics Committee (CBNU 2016-61). Immature male ICR mice (Damul Science, Suwon, Korea) at postnatal 5–21 days were used, and were housed under 12-h light/dark cycles with free access to water and food.
Brain slice preparation
Mice were decapitated between 10:00 and 14:00, the brains rapidly removed and coronal brainstem slices (250–300 µM thickness) containing the Vc were prepared with a vibratome (Microm, Walldorf, Germany) in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) 126 NaCl, 2.5 KCl, 2.4 CaCl2, 1.2 MgCl2, 11 d-glucose, 1.4 NaH2PO4, and 25 NaHCO3 (pH 7.4, bubbled with 95% O2 and 5% CO2). Slices were allowed to recover in oxygenated ACSF for at least 1 h at room temperature.
Electrophysiology
Slices were transferred to the recording chamber, submerged, and continuously superfused with oxygenated ACSF at a flow rate of 4–5 ml/min. Each slice was viewed on the stage of an upright microscope (BX51WI, Olympus, Tokyo, Japan) equipped with differential interference contrast optics. Patch pipettes were pulled from borosilicate glass capillary tubing (PG52151-4, WPI, Sarasota, FL, USA) on a Flaming/Brown micropipette puller (P-97; Sutter Instruments Co., Novato, CA, USA), and filled with solution containing (in mM) 140 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 4 Mg-ATP, and 10 EGTA (pH 7.3 adjusted with KOH). The tip resistances of the recording electrodes were between 4 and 6 MΩ. Offset potentials were compensated directly before gigaseal formation. After a GΩ seal was formed with an SG neuron, the cell membrane patch was ruptured by negative pressure, and then, whole-cell patch clamp recording was performed.
Signals were amplified with an Axopatch 200B (Axon Instruments, San Francisco, CA, USA), filtered at 2 kHz with a Bessel filter (Axon Instruments), and digitized at 1 kHz using the Digidata 1440A (Axon Instruments). Data were collected and analyzed with Clampex 10.2 software (Axon Instruments), MiniAnalysis (ver 6.0.7; Synaptosoft Inc.) and Origin 8 software (OriginLab Corp., Northampton, MA, USA). All recordings were performed at room temperature. The whole cell patch recordings were made from visually identified SG neurons under voltage clamp mode, in which the holding potential was −60 mV.
Chemicals and statistical analysis
BoNT/A (Botulax®) kindly provided by Hugel Inc. (Chuncheon, Republic of Korea); d,l-2-amino-5-phosphonopentanoic acid (AP5), 6-Cyano-7-nitroquinopentanoic (CNQX) and tetrodotoxin citrate (TTX) were purchased from Tocris Bioscience (Bristol, UK) and chemicals for ACSF were purchased from Sigma-Aldrich (St. Louis, MO, USA). Stock solutions of all drugs were dissolved in distilled water except BoNT/A which was dissolved in 0.9% NaCl. All stock solutions were diluted to working concentrations using ACSF immediately before use and applied by superfusion. Pretreated chemicals were applied for 3–4 min before the application of a solution containing both pretreated chemicals and BoNT/A. Miniature postsynaptic currents (mPSCs) were obtained by application of 0.5 µM tetrodtoxin (TTX), a voltage-gated Na+ channel blocker. Spontaneous inhibitory postsynaptic currents (sIPSCs) were isolated using CNQX (10 µM) and AP5 (20 µM), ionotropic glutamate receptor antagonists.
The PSCs were analyzed using Mini-Analysis software. The peak detection criteria were set at >10 pA of amplitude threshold, <8 ms of rise-time, and >5 ms of decay time of sPSCs. Any uncounted synaptic currents were detected manually. A paired t-test was used to compare the means between two groups. A Kolmogorov-Smirnov (K-S) test was used to compare the frequency and amplitude of PSCs in a single neuron. One-way ANOVA and Pearson's Chi-square test were used to compare the means more than two groups and the proportion of each response, respectively. All values were expressed as the mean±SEM and p<0.05 was considered significant.
RESULTS
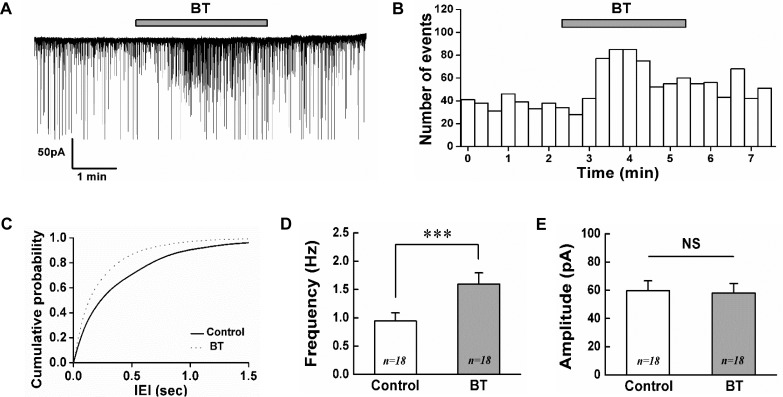
To evaluate the effect of BoNT/A on SG neurons of the Vc, electrophysiological recordings were obtained from total 66 SG neurons in voltage clamp mode. A bath application of BoNT/A (BT, 3 nM) increased the frequency of spontaneous postsynaptic currents (sPSCs) in 18 (37.5%) of the 48 neurons tested. Out of the remaining neurons, 5 (10.4%) neurons showed decrease of sPSCs frequency and 25 (52.1%) neurons were not affected by BoNT/A (3 nM). Fig. 1A shows a representative trace showing the frequency increase of sPSCs by BoNT/A (3 nM). The time course histogram of sPSCs frequency also indicates an increase by the application of BoNT/A (Fig. 1B). Fig. 1C shows the cumulative probability curve of the inter-event intervals (IEI) of sPSCs was left-shifted by BoNT/A (K-S test, p<0.001). The mean frequency of sPSCs in the presence of 3 nM BoNT/A (1.60±0.20 Hz, n=18) was significantly increased compared to control (0.94±0.14 Hz, n=18, paired t-test, p<0.001, Fig. 1D). However, the mean amplitude of sPSCs was not affected by 3 nM BoNT/A (58.1±6.69 pA, n=18) compared to control (59.6±7.08 pA, n=18, paired t-test, p>0.05, Fig. 1E).
Fig. 1. BoNT/A increases the frequency of sPSCs on SG neurons.
(A) A representative trace showing the frequency increase of sPSCs by 3 nM BoNT/A. (B) Time course frequency histogram (bin size 20 s) of the current trace in (A). (C) Cumulative probability histogram of the inter-event interval (IEI) of sPSCs in the control (solid line) and with BoNT/A (BT, dotted line). Bar graphs showing the mean frequency (D) and mean amplitude (E) of sPSCs in control and BoNT/A, respectively. Values represent mean±SEM, *** represents p<0.001, NS (not significant), BT (BoNT/A).
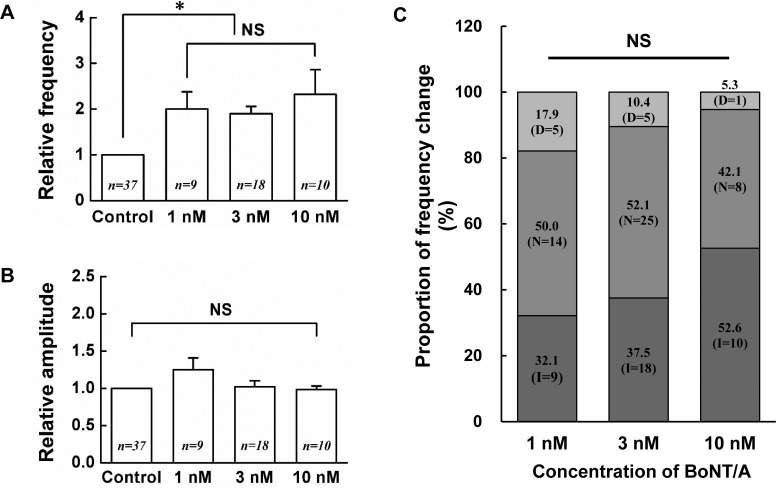
Next, concentration dependency of BoNT/A was tested in SG neurons at the concentration of 1, 3 and 10 nM. When 1 nM BoNT/A was bath applied, the frequency increase of sPSCs in 9 (32.1%) of the 28 SG neurons tested. However, 5 (17.9%) neurons showed the decrease of sPSCs frequency and 14 (50%) neurons were not affected by BoNT/A (1 nM). Similarly, when 10 nM BoNT/A was applied, the frequency increase of sPSCs was shown in 10 (52.6%) of the 19 SG neurons tested. Remaining 1 (5.3%) and 10 (42.1%) neurons showed the decrease and no change of sPSCs frequency, respectively. Fig. 2A shows the relative mean frequency changes by 1 nM (2.00±0.38, n=9, p<0.05), 3 nM (1.90±0.16, n=18, p<0.01) and 10 nM (2.32±0.54, n=10, p<0.001) BoNT/A on SG neurons which increased the frequency of sPSCs compared to control. Among 1, 3 and 10 nM concentrations of BoNT/A, no significant difference was observed in the relative mean frequencies of sPSCs by BoNT/A. Fig. 2B shows the relative mean amplitude changes by 1 nM (1.25±0.16, n=9, p>0.05), 3 nM (1.02±0.08, n=18, p>0.05) and 10 nM (0.98±0.05, n=10, p>0.05) BoNT/A on the SG neurons which increased the frequency of sPSCs by BoNT/A. Fig. 2C shows the proportion of frequency variation by BoNT/A. At each concentration, the proportion of the increase, no change and decrease in the frequency of sPSCs caused by BoNT/A was not statistically significant (χ2-test, p>0.05).
Fig. 2. Concentration-response relationship of BoNT/A on the SG neurons.
Each bar graphs show the relative mean frequency (A) and relative mean amplitude (B) of sPSCs by application of 1, 3, 10 nM BoNT/A compared to control, respectively. Values represent mean±SEM, (C) Stack column graph showing the proportion of frequency change by BoNT/A (1, 3, 10 nM). The numbers in parentheses mean the number of neurons recorded. D (decrease), N (no change), I (increase), * (p<0.05), NS (not significant).
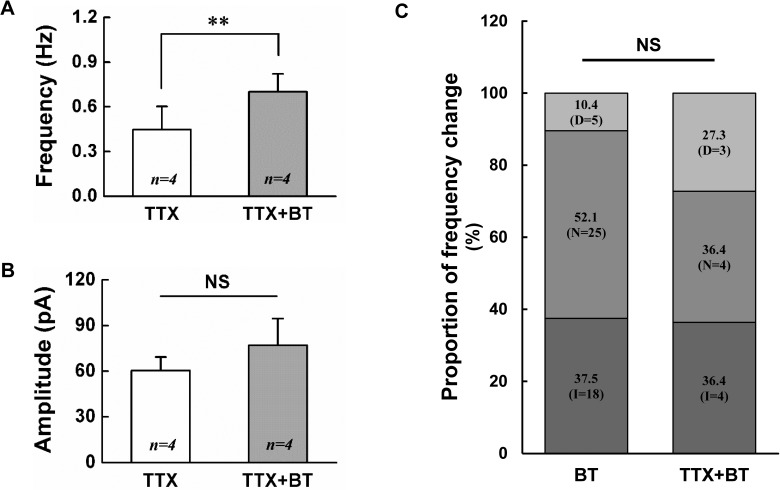
To clarify that BoNT/A-induced frequency increase is action potential dependent, BoNT/A (3 nM) was applied in the presence of 0.5 µM TTX, a voltage-gated Na+ channel blocker which blocks action potential dependent transmission. When 3 nM BoNT/A was applied in the presence of TTX, the frequency increase of mPSCs was shown in 4 (36.4%) of the 11 SG neurons tested. Remaining 3 (27.3%) and 4 (36.4%) neurons showed the decrease and no change of mPSCs frequency, respectively. The mean frequency of mPSCs by 3 nM BoNT/A (0.70±0.12 Hz, n=4, p<0.05) in the presence of TTX was significantly increased compared to TTX alone (0.45±0.16 Hz, n=4, paired t-test, p<0.05, Fig. 3A). However, the mean amplitude of mPSCs was not affected by 3 nM BoNT/A (77.0±17.60 pA, n=4) compared to control (60.3±8.99 pA, n=4, paired t-test, p>0.05, Fig. 3B). Fig. 3C shows the proportion of frequency variation by 3 nM BoNT/A in the absence and the presence of TTX. The proportion of the increase, no change and decrease in the frequency caused by BoNT/A was not statistically significant between the absence and the presence of TTX (χ2-test, p>0.05). These results indicate that BoNT/A-mediated increase of sPSCs frequency at least partly is not action potential dependent.
Fig. 3. BoNT/A increases the frequency of mPSCs on SG neurons.
Bar graphs show the mean frequency (A) and mean amplitude (B) of sPSCs by application of 3 nM BoNT/A alone (BT) and BT in the presence of 0.5 µM TTX (TTX+BT), respectively. (C) Stack column graph showing the proportion of frequency change between 3 nM BT and BT in the presence of TTX (TTX+BT). D (decrease), N (no change), I (increase), * (p<0.05), NS (not significant).
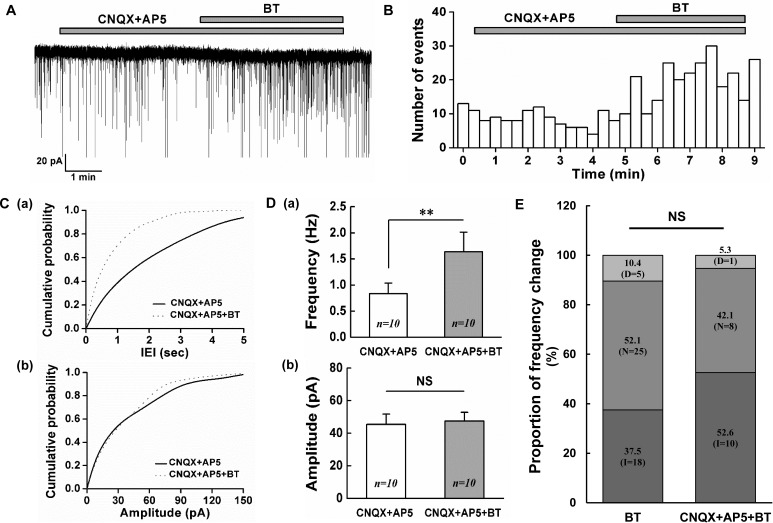
To examine inhibitory neurotransmitters are involved in the BoNT/A-mediated frequency increase of sPSCs, BoNT/A (3 nM) was bath applied in the presence of CNQX (10 µM), a non-NMDA glutamate receptor antagonist with AP5 (20 µM), an NMDA glutamate receptor antagonist. When 3 nM BoNT/A was applied in the presence of CNQX with AP-5, the frequency increase of sIPSCs was shown in 10 (52.6%) of the 19 SG neurons tested. Remaining 1 (5.3%) and 8 (42.1%) neurons showed the decrease and no change of sIPSCs frequency, respectively. Fig. 4A shows a representative trace of the frequency increase of sIPSCs by BoNT/A (3 nM). The time course histogram of sIPSCs frequency also indicates an increase of frequency by the application of BoNT/A (3 nM, Fig. 4B). The cumulative probability curve of the IEI of sIPSCs was left shifted (K-S test, p<0.05, Fig. 4C-a) suggesting the increase of the frequency of sIPSCs but not that of amplitude (Fig. 4C-b) by BoNT/A (3 nM). The mean frequency of sIPSCs by 3 nM BoNT/A (1.64±0.37 Hz, n=10) in the presence of CNQX with AP5 was significantly increased compared to that of CNQX with AP5 (0.83±0.20 Hz, n=10, paired t-test, p<0.01, Fig. 4D-a). However, the mean amplitude of sIPSCs was not affected by 3 nM BoNT/A (47.5±5.46 pA, n=10) compared to that of CNQX with AP5 (45.5±6.25 pA, n=10, paired t-test, p>0.05, Fig. 4D-b). Fig. 4E shows the proportion of frequency variation by 3 nM BoNT/A in the absence and the presence of CNQX with AP5. The proportion of the increase, no change and decrease in the frequency caused by BoNT/A was not statistically significant between the absence and the presence of CNQX with AP5 (χ2-test, p>0.05). These results suggest that BoNT/A can induce the release of inhibitory neurotransmitters on the SG area of the Vc at least partly.
Fig. 4. BoNT/A increases the frequency of sIPSCs on SG neurons.
(A) A representative trace showing the frequency increase of sIPSCs by 3 nM BoNT/A (BT) in the presence of CNQX (10 µM) and AP5 20 µM. (B) Time course frequency histogram (bin size 20 s) of the sPSCs of the current trace in (A). (C) Cumulative probability histograms of the inter-event interval (IEI, a) and amplitude (b) of the sPSCs of the current trace in (A). Solid line (CNQX+AP5), Dotted line (CNQX+AP5+BT) (D) Comparisons of the mean frequency (a) and mean amplitude (b) of sPSCs between CNQX+AP5 and 3 nM BoNT/A in the presence of CNQX+AP5, respectively. (E) Stack column graph showing the proportion of frequency change by 3 nM BoNT/A alone and BoNT/A in the presence of CNQX+AP5. D (decrease), N (no change), I (increase). ** (p<0.01), NS (not significant).
DISCUSSION
In this study, we report that bath application of BoNT/A increases sPSCs frequency at least partly without changing the amplitude on the SG neurons of the Vc in immature mice and the BoNT/A-mediated frequency increase of sPSCs was not affected by TTX and persisted in the presence of CNQX and AP5, ionotropic glutamate receptor blockers.
BoNT/A is commonly known to act via cleavage of SNAP-25, preventing the correct SNARE complex assembly that mediates synaptic vesicle fusion with presynaptic membrane, thereby inhibiting acetylcholine release [19]. Based on this mode of action, it has been suggested that BoNT/A could be used to treat various neuromuscular and autonomous disorders [20,21]. Recently, many studies reported that peripherally applied BoNT/A can be transported along the axon in a retrograde manner [15,22,23,24] and it has been suggested that BoNT/A can modulate neuronal activities in the CNS [22,25,26]. Although SNAP-25 cleavage might be well known mechanism of BoNT/A-mediated action, the other central actions are not clearly elucidated yet.
In this study, when BoNT/A (3 nM) was bath applied, 37.5% of SG neurons tested showed the frequency increase of sPSCs. On the contrary, the decrease or no change of sPSCs were shown in 10.4% and 52.1% neurons tested, respectively. Similar proportions of response were observed by the application of BoNT/A at concentrations of 1 and 10 nM. It has been suggested that SG neurons do not project to higher brain centers, unlike other neurons in lamina 1 and deeper laminae, so most SG neurons are interneurons for processing of nociceptive information [27] and the SG is composed of various neuronal types. For example, the SG neurons of the Vc were shown four firing patterns by depolarizing current injection, such as phasic (34%), tonic (39%), delayed (16%) and single spiking (11%) patterns in mice [28]. So, this heterogeneous composition of the SG neurons of the Vc may explain why the frequency increase of sPSCs by BoNT/A was seen only in 38% of neurons tested. Similarly, only 36.4% of neurons tested, BoNT/A (3 nM) increased the frequency of sPSCs in the presence of tetrodotoxin, a voltage sensitive sodium channel blocker. Although observed at a relatively low proportion, it can be suggested that BoNT/A increases the frequency of mPSCs in a similar way to that of sPSCs at least partly. This result indicates that BoNT/A-mediated increase of sPSCs frequency is not action potential dependent suggesting that BoNT/A can act on the presynaptic terminals for neurotransmitter release. In addition, when 3 nM BoNT/A was applied, the majority (52.6%) of neurons tested showed the increase of sIPSCs frequency without amplitude change. This result indicates that BoNT/A can induce the release of inhibitory neurotransmitters on the SG area of the Vc.
These main results of our study may sound paradoxical because a number of studies have shown that BoNT/A prevents the release of neurotransmitters [29,30,31,32]. However, a significant aspect of these results relates to the time post of BoNT/A action. In this study, we bath applied BoNT/A less than five minutes. The frequency increases of mPSCs and sIPSCs by BoNT/A in this study are consistent with previous studies showing relatively rapid action of BoNT/A. According to a study using mouse embryonic stem cell-derived neurons, the application of BoNT/A caused a biphasic response in mPSCs with an initial (30–49 minutes) increase of frequency and followed by a gradual frequency decrease [33]. It has also been demonstrated that low concentration of BoNT/A initially increased the frequency of mIPSCs and was gradually supressed in sacral dorsal commissural nucleus neurons of rat [34].
These results indicate that there could be other unknown mechanisms besides SNAP-25 cleavage by BoNT/A. Some of the evidence for this hypothesis has been reported. For example, bicuculline, a GABAA receptor antagonist prevented BoNT/A-induced antinociception against pain induced by formalin injection and mechanical allodynia by sciatic nerve transection suggesting that BoNT/A can interact with GABAergic transmission at the spinal level [35]. Since GABA is a principal inhibitory neurotransmitter, the preventing of BoNT/A-mediated antinociception by bicuculline may be interpreted as BoNT/A can increase GABA release at least partly. Our results can provide an evidence to solve the puzzle how GABAergic transmission is activated. Also, the conversion of total SNAP-25 to the BoNT/A-cleaved product, which functionally preventing neurotransmitter release, was detected for the first time in 2 h followed by a progressive increase [33]. Furthermore, it was also suggested that mature GABAergic nerve terminals probably lack SNAP-25 [36].
In this study, we used immature mice (postnatal day, PND5-21). A number of studies have suggested that nociception is age dependent [37,38,39] and spontaneous GABA release is also gradually increased over postnatal days [40]. For example, the error rate of heat-nociceptive withdrawal reflex is very high up to PND10 and then gradually reduced by PND21 to adult levels in rats [37]. These results suggest that there may also be a variety of age-dependent responses by BoNT/A on sIPSCs, which requires further studies to identify them. In addition, the Vc is also called the medullary dorsal horn because its structure and function are similar to the spinal dorsal horn. So, it is further required to investigate the action of BoNT/A of the spinal dorsal horn, which functions similarly to the Vc.
Taken together, the present study demonstrated that bath application of BoNT/A increases sIPSCs frequency on the SG neurons of the Vc which play a key role to modulate orofacial pain processing in immature mice. Although BoNT/A-mediated action is not a typical pattern, this is the first report to show rapid action of BoNT/A on SG neurons of the Vc in mice. Therefore, the results can provide a clue to reveal unknown action of BoNT/A in controlling orofacial pain at CNS level.
ACKNOWLEDGEMENTS
This research was supported by the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2016R1D1A3B03932241, NRF-2017R1A5A2015391) and by Hugel Inc. (Chuncheon, Republic of Korea).
Footnotes
Author contributions: S.H.J.; performed experiments and analysis, S.J.P.; reviewed data, C.J.L.; contributed materials, D.K.A. and S.K.H.; conceptualized and designed the studies, S.H.J. and S.K.H.; drafted and wrote the final manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000;43:249–259. doi: 10.1067/mjd.2000.105567. [DOI] [PubMed] [Google Scholar]
- 2.Pellizzari R, Rossetto O, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philos Trans R Soc Lond B Biol Sci. 1999;354:259–268. doi: 10.1098/rstb.1999.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson LL. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981;33:155–188. [PubMed] [Google Scholar]
- 4.Peng Chen Z, Morris JG, Jr, Rodriguez RL, Shukla AW, Tapia-Núñez J, Okun MS. Emerging opportunities for serotypes of botulinum neurotoxins. Toxins (Basel) 2012;4:1196–1222. doi: 10.3390/toxins4111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–766. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 6.Kim DW, Lee SK, Ahnn J. Botulinum toxin as a pain killer: players and actions in antinociception. Toxins (Basel) 2015;7:2435–2453. doi: 10.3390/toxins7072435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavone F, Luvisetto S. Botulinum neurotoxin for pain management: insights from animal models. Toxins (Basel) 2010;2:2890–2913. doi: 10.3390/toxins2122890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brin MF, Binder WJ, Blitzer A, Schenrock L, Pogoda JM. Botulinum toxin type A for pain and headache. In: Brin MF, Hallett M, Jankovic J, editors. Scientific and therapeutic aspects of botulinum toxin. 1st ed. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 233–250. [Google Scholar]
- 9.Wheeler AH. Therapeutic uses of botulinum toxin. Am Fam Physician. 1997;55:541–545. [PubMed] [Google Scholar]
- 10.Truong DD, Jost WH. Botulinum toxin: clinical use. Parkinsonism Relat Disord. 2006;12:331–355. doi: 10.1016/j.parkreldis.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Bach-Rojecky L, Lacković Z. Antinociceptive effect of botulinum toxin type a in rat model of carrageenan and capsaicin induced pain. Croat Med J. 2005;46:201–208. [PubMed] [Google Scholar]
- 12.Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004;107:125–133. doi: 10.1016/j.pain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Luvisetto S, Marinelli S, Lucchetti F, Marchi F, Cobianchi S, Rossetto O, Montecucco C, Pavone F. Botulinum neurotoxins and formalin-induced pain: central vs. peripheral effects in mice. Brain Res. 2006;1082:124–131. doi: 10.1016/j.brainres.2006.01.117. [DOI] [PubMed] [Google Scholar]
- 14.Lee WH, Shin TJ, Kim HJ, Lee JK, Suh HW, Lee SC, Seo K. Intrathecal administration of botulinum neurotoxin type A attenuates formalin-induced nociceptive responses in mice. Anesth Analg. 2011;112:228–235. doi: 10.1213/ANE.0b013e3181ffa1d7. [DOI] [PubMed] [Google Scholar]
- 15.Bach-Rojecky L, Lacković Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Behav. 2009;94:234–238. doi: 10.1016/j.pbb.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- 17.Cervero F, Iggo A. The substantia gelatinosa of the spinal cord: a critical review. Brain. 1980;103:717–772. doi: 10.1093/brain/103.4.717. [DOI] [PubMed] [Google Scholar]
- 18.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matak I, Lacković Z. Botulinum toxin A, brain and pain. Prog Neurobiol. 2014;119–120:39–59. doi: 10.1016/j.pneurobio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler A, Smith HS. Botulinum toxins: mechanisms of action, antinociception and clinical applications. Toxicology. 2013;306:124–146. doi: 10.1016/j.tox.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Dressler D. Botulinum toxin therapy: its use for neurological disorders of the autonomic nervous system. J Neurol. 2013;260:701–713. doi: 10.1007/s00415-012-6615-2. [DOI] [PubMed] [Google Scholar]
- 22.Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28:3689–3696. doi: 10.1523/JNEUROSCI.0375-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matak I, Bach-Rojecky L, Filipović B, Lacković Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience. 2011;186:201–207. doi: 10.1016/j.neuroscience.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Filipović B, Matak I, Bach-Rojecky L, Lacković Z. Central action of peripherally applied botulinum toxin type A on pain and dural protein extravasation in rat model of trigeminal neuropathy. PLoS One. 2012;7:e29803. doi: 10.1371/journal.pone.0029803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HJ, Lee GW, Kim MJ, Yang KY, Kim ST, Bae YC, Ahn DK. Antinociceptive effects of transcytosed botulinum neurotoxin type A on trigeminal nociception in rats. Korean J Physiol Pharmacol. 2015;19:349–355. doi: 10.4196/kjpp.2015.19.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Restani L, Antonucci F, Gianfranceschi L, Rossi C, Rossetto O, Caleo M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A) J Neurosci. 2011;31:15650–15659. doi: 10.1523/JNEUROSCI.2618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guy N, Chalus M, Dallel R, Voisin DL. Both oral and caudal parts of the spinal trigeminal nucleus project to the somatosensory thalamus in the rat. Eur J Neurosci. 2005;21:741–754. doi: 10.1111/j.1460-9568.2005.03918.x. [DOI] [PubMed] [Google Scholar]
- 28.Davies AJ, North RA. Electrophysiological and morphological properties of neurons in the substantia gelatinosa of the mouse trigeminal subnucleus caudalis. Pain. 2009;146:214–221. doi: 10.1016/j.pain.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 29.McMahon HT, Foran P, Dolly JO, Verhage M, Wiegant VM, Nicholls DG. Tetanus toxin and botulinum toxins type A and B inhibit glutamate, gamma-aminobutyric acid, aspartate, and met-enkephalin release from synaptosomes. Clues to the locus of action. J Biol Chem. 1992;267:21338–21343. [PubMed] [Google Scholar]
- 30.Durham PL, Cady R, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache. 2004;44:35–42. doi: 10.1111/j.1526-4610.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- 31.Morris JL, Jobling P, Gibbins IL. Botulinum neurotoxin A attenuates release of norepinephrine but not NPY from vasoconstrictor neurons. Am J Physiol Heart Circ Physiol. 2002;283:H2627–H2635. doi: 10.1152/ajpheart.00477.2002. [DOI] [PubMed] [Google Scholar]
- 32.Nakov R, Habermann E, Hertting G, Wurster S, Allgaier C. Effects of botulinum A toxin on presynaptic modulation of evoked transmitter release. Eur J Pharmacol. 1989;164:45–53. doi: 10.1016/0014-2999(89)90229-x. [DOI] [PubMed] [Google Scholar]
- 33.Beske PH, Scheeler SM, Adler M, McNutt PM. Accelerated intoxication of GABAergic synapses by botulinum neurotoxin A disinhibits stem cell-derived neuron networks prior to network silencing. Front Cell Neurosci. 2015;9:159. doi: 10.3389/fncel.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akaike N, Ito Y, Shin MC, Nonaka K, Torii Y, Harakawa T, Ginnaga A, Kozaki S, Kaji R. Effects of A2 type botulinum toxin on spontaneous miniature and evoked transmitter release from the rat spinal excitatory and inhibitory synapses. Toxicon. 2010;56:1315–1326. doi: 10.1016/j.toxicon.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Drinovac V, Bach-Rojecky L, Lacković Z. Association of antinociceptive action of botulinum toxin type A with GABA-A receptor. J Neural Transm (Vienna) 2014;121:665–669. doi: 10.1007/s00702-013-1150-6. [DOI] [PubMed] [Google Scholar]
- 36.Matteoli M, Pozzi D, Grumelli C, Condliffe SB, Frassoni C, Harkany T, Verderio C. The synaptic split of SNAP-25: different roles in glutamatergic and GABAergic neurons? Neuroscience. 2009;158:223–230. doi: 10.1016/j.neuroscience.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Waldenström A, Thelin J, Thimansson E, Levinsson A, Schouenborg J. Developmental learning in a pain-related system: evidence for a cross-modality mechanism. J Neurosci. 2003;23:7719–7725. doi: 10.1523/JNEUROSCI.23-20-07719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz-Medina J, Baulies A, Bura SA, Valverde O. Paclitaxel-induced neuropathic pain is age dependent and devolves on glial response. Eur J Pain. 2013;17:75–85. doi: 10.1002/j.1532-2149.2012.00172.x. [DOI] [PubMed] [Google Scholar]
- 39.Park SA, Yang EJ, Han SK, Park SJ. Age-related changes in the effects of 5-hydroxytryptamine on substantia gelatinosa neurons of the trigeminal subnucleus caudalis. Neurosci Lett. 2012;510:78–81. doi: 10.1016/j.neulet.2011.12.069. [DOI] [PubMed] [Google Scholar]
- 40.Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci. 2004;24:4749–4757. doi: 10.1523/JNEUROSCI.5211-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]