Fig. 3.
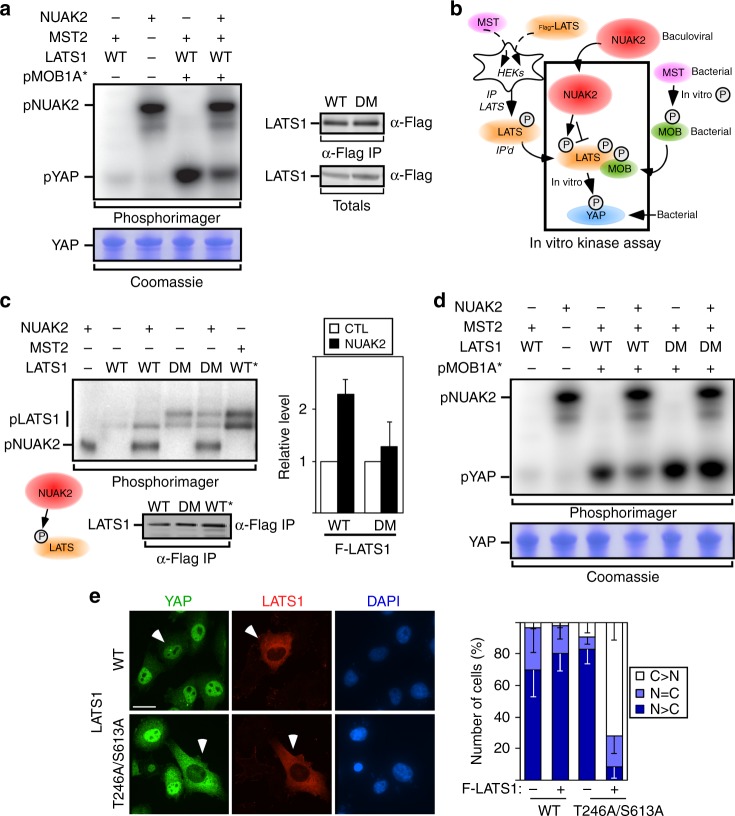
NUAK2 phosphorylates and inhibits LATS activity. a Purified NUAK2 blocks LATS-mediated phosphorylation of YAP in an in vitro kinase assay. WT wild type, pMOB1A* the modified active variant (T12 to T353) of MOB1A (see Methods). Levels of immunoprecipitated LATS used for a and d are shown (right). b A schematic depicting in vitro kinase assay. The method used to produce and activate each component is indicated. c NUAK2 phosphorylates the wild-type (WT) but not the T246/S613 double mutant (DM) LATS in an in vitro kinase assay. Levels of immunoprecipitated WT and DM LATS are comparable. Relative phosphorylation levels from blots is quantitated and plotted as the mean ± SD (n = 5) (right). WT* MST-activated WT LATS. Note that the DM mutant migrates as a doublet, similar to MST-activated WT LATS, consistent with the notion that endogenous NUAKs have a reduced ability to block LATS1 activation. d NUAK2 does not inhibit the activity of the LATS T246/S613 double mutant (DM) in an in vitro kinase assay. e Expression of LATS T246/S613 (DM) induces cytoplasmic localization of YAP in MDA-MB231 cells. Quantitated results are plotted as the mean ± SD (n = 3). Scale bar, 25 μm. N: nuclear, C: cytoplasmic