Figure 1.
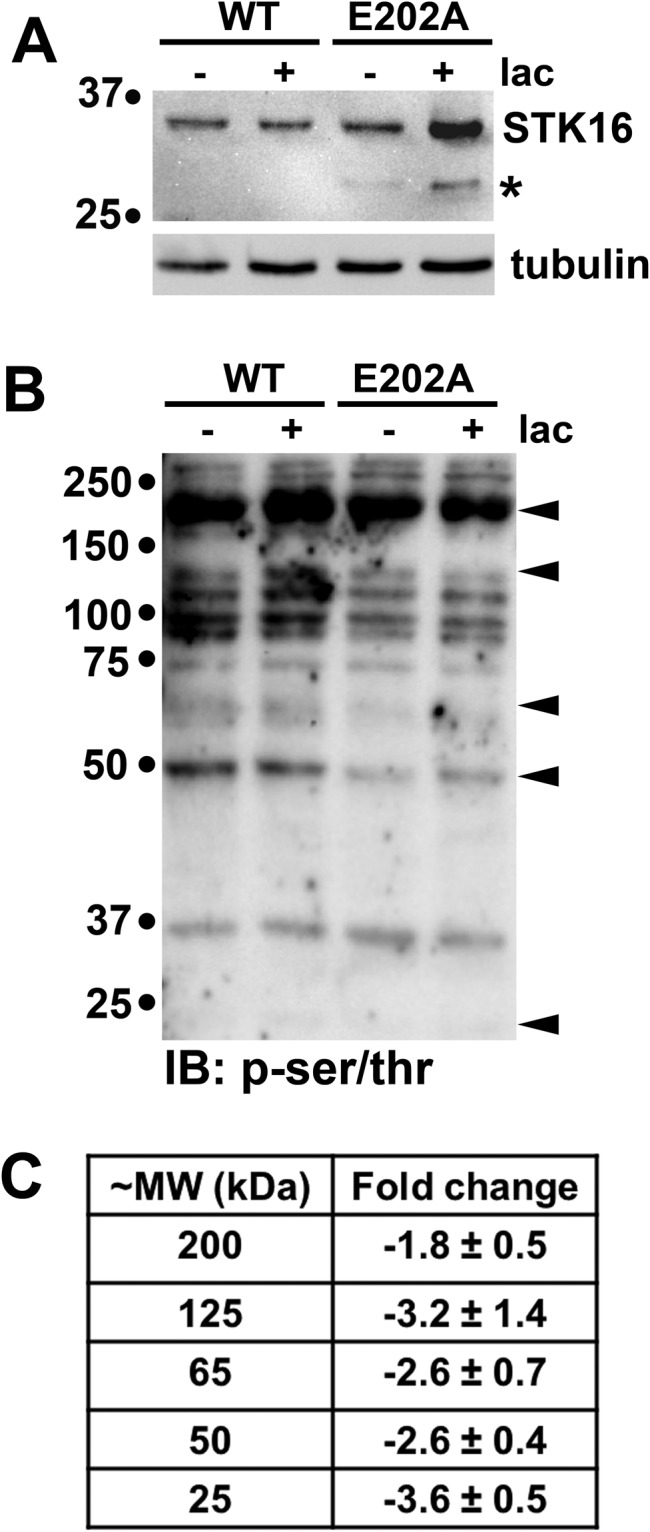
E202A expression leads to decreased serine/threonine phosphorylation in total cell lysates. (A) Total cell lysates were prepared from WIF-B cells expressing wild type (WT) or the kinase dead STK16 (E202A) after treatment in the absence or presence of 5 μM lactacystin (lac) for 4 h at 37 °C. Lysates (20 μg total protein per lane) were immunoblotted for STK16 (with anti-V5 antibodies) (top panel) or α-tubulin (as a loading control). The asterisk marks a known degradative species of the mutant kinase. Molecular weight standards are indicated on the left (in kDa). (B) The lysates as in A were immunoblotted (IB) with antibodies against phosphorylated-serine/threonine residues (p-ser/thr). Molecular weight standards are indicated on the left (in kDa) and arrows on the right indicate proteins with decreased immunoreactivity in E202A expressing cells. (C) The fold-decrease in immunoreactivity of the proteins marked with arrows in B (from samples without lactacystin) was determined using densitometry. Values represent the average from three independent experiments ± SEM.
