Abstract
Brain rhythms are widely believed to reflect numerous cognitive processes. Changes in rhythmicity have been associated with pathological states. However, the mechanism underlying these rhythms remains unknown. Here, we present a theoretical analysis of the evolvement of rhythm generating capabilities in neuronal circuits. We tested the hypothesis that brain rhythms can be acquired via an intrinsic unsupervised learning process of activity dependent plasticity. Specifically, we focused on spike timing dependent plasticity (STDP) of inhibitory synapses. We detail how rhythmicity can develop via STDP under certain conditions that serve as a natural prediction of the hypothesis. We show how global features of the STDP rule govern and stabilize the resultant rhythmic activity. Finally, we demonstrate how rhythmicity is retained even in the face of synaptic variability. This study suggests a role for inhibitory plasticity that is beyond homeostatic processes.
Introduction
Rhythmic activity has been reported to be related to a range of cognitive processes including the encoding of external stimuli, attention, learning and consolidation of memory1–5. In certain cases, changes in rhythmicity have been associated with pathological states6–8. Numerous theoretical studies have investigated various mechanisms that may produce these rhythmic behaviors. All these mechanisms assume specific parameter ranges, such as the mean synaptic strength between different neuronal populations. Deviations from the assumed parameter range results in drastic changes to rhythmic activity. However, the underlying mechanism that allows the synaptic weights, for example, to evolve into a state of rhythmic activity, and then to choose and stabilize a specific rhythm still remains enigmatic. What also remains unclear is whether this mechanism can be based on activity dependent plasticity9,10.
Here we consider a specific type of activity dependent plasticity known as spike timing dependent plasticity (STDP). STDP can be thought of as an extension of Hebb’s rule11 to the temporal domain that takes the effect of the causal relationship between pre- and post-synaptic firing on the potentiation and depression of the synapse into account. STDP has been identified in various systems in the brain, and a rich repertoire of causal relations has been described12–22.
Considerable theoretical efforts have been devoted to investigating the possible computational implications of STDP23–41. It was shown that Hebbian STDP of excitatory synapses can give rise to the emergence of response selectivity at the level of the post-synaptic neuron by inducing competition between correlated subgroups of input neurons24,25,30. For example, in the visual system, modeling studies have shown how spatial correlations together with STDP can develop response selectivity in the form of ocular dominance and directional selectivity24,27,42–46. On the other hand, Hebbian STDP of inhibitory synapses may provide a homeostatic mechanism that can balance the excitatory and inhibitory inputs to the cell17,35,39,47,48.
Oscillatory activity may have a strong effect on STDP as oscillations cause neurons to fire repeatedly with distinct spike timing relations. In the context of development, oscillations and repeated spatiotemporal patterns of activity may play an important role in shaping emergent neuronal connectivity maps49,50. The effect and possible computational role of rhythmic activity on STDP has been addressed in several studies51–60. However, in all of these studies the rhythmic activity was either an inherent property of the neuron or inherited via feed-forward connections from inputs that were already oscillating.
Can STDP contribute to the development of temporal structure in the neuronal response? In a recent work it was shown that STDP can contribute to synchronization in a network of interneurons oscillating in the gamma frequency61. It was further shown that STDP can facilitate the propagation of synchronous activity62. A numerical study simulating a large scale detailed thalamocortical model argued that oscillations may emerge with STDP63. However, the principles that underlie the emergence of oscillations with STDP remain unclear.
Here we investigated whether rhythmic behavior can emerge via a process of STDP, and if so – under what conditions, and how the features of the STDP rule govern the resultant rhythmic activity? We addressed these fundamental questions in a modelling study. We chose to study STDP dynamics using the framework of a simplified toy-model of two competing inhibitory populations with reciprocal inhibition. The choice of the toy-model was made primarily to enable complete analytical parsing. Nevertheless, it is important to note that this type of architecture has been widely used to model winner-takes-all computation and decision making64–69, has been observed in various brain regions70–72, and was recently suggested to implement a latency code readout mechanism for fast decisions73. Short term plasticity was also incorporated into our model in the form of firing rate adaptation in a manner that is similar to that of74,75 to enable a richer dynamical structure for the neuronal responses76–78. As we are interested in obtaining as complete analytical understanding we primarily focus on the most compact model description of reciprocal inhibition with the smallest number of parameters.
Below, we first define the dynamical model for the neuronal responses and analyze it for fixed synaptic weights. This analysis provides the phase diagram of the system, which depicts the different possible dynamical states of the network as a function of the synaptic weights. Next, we introduce STDP. STDP induces a flow along the phase diagram of the system, by allowing the synaptic weights themselves to evolve according to the plasticity rule in an activity dependent manner. This flow is then analyzed in the limit of slow learning. We show that under a broad range of parameters STDP can generate and stabilize oscillatory activity in the brain, and, that this oscillatory activity can be governed by global features of the STDP rule. Finally, we summarize our results and discuss possible outcomes and extensions to the simplified model studied here.
Results
The neuronal response model
We explored STDP dynamics in a model of two neuronal populations with reciprocal inhibition. The spiking activity of individual neurons in each population was modelled as an inhomogeneous Poisson process with a mean firing rate that obey the following dynamics:
| 1 |
| 2 |
| 3 |
| 4 |
where Ni is the number of neurons in population i = 1, 2, rix is the firing rate of neuron x in population i that receives external excitatory input Ii. For simplicity we take I1 = I2 ≡ I. Throughout this paper, the function g(x) will be taken to be a threshold linear function of its input, for x > 0 and 0 otherwise (see also79). The term aix represents the adaptation variable of neuron x in population i, and parameter A denotes the adaptation strength. Jix,jy ≥ 0 is the strength of the inhibitory coupling from neuron y in population j to neuron x in population i.
Parameter τm is the membrane time constant and τa is the time constant of the adaptation. It is assumed that adaptation is a slower process than the neural response to its input, τa > τm. Thus, the neuronal firing rate follows changes in its input with a time scale of τm and then adapts its rate in response to a constant input with a time scale of τa by decreasing its firing rate by a factor of 1 + A. We further assume, for simplicity, that the populations are relatively homogeneous. Thus, we omit the sub-indices x and y from Eqs (1–4). ri represents the mean activity in population i, and Jij the mean synaptic weight from a pre-synaptic neuron in population j to a post-synaptic neuron in population i, see Eqs (21–24) in Methods. In the limit of slow adaptation, , a complete analytical solution is possible; see the phase diagram section and the limit cycle calculations in Methods. Unless noted otherwise the results are given in the limit and time is measured in units of the adaptation time constant, τa.
The phase diagram
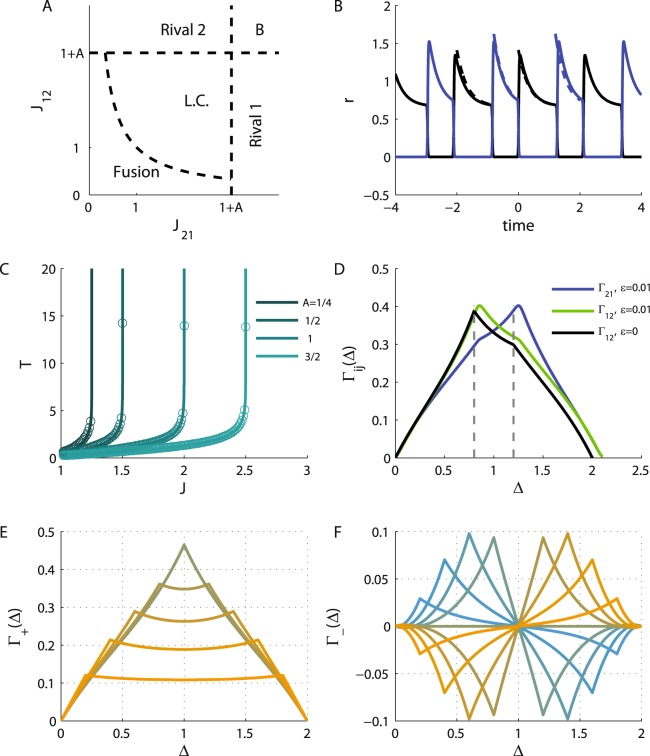
Figure 1A depicts the phase diagram of the model in the plane of J12 and J21. If the inhibition from population 1 to population 2, J21, is sufficiently strong relative to the adaptation, J21 > 1 + A, there exists a fixed point solution that we term Rival 1, in which population 1 fully suppresses population 2 (r2 = 0). Similarly, the Rival 2 solution, in which population 2 fully suppresses population 1, exists for J12 > 1 + A. The Rival states are stable wherever they exist and may also co-exist (this bi-stable solution is denoted by B on the phase diagram); see the fixed points of the dynamics section in Methods.
Figure 1.
Neuronal dynamics. (A) The phase diagram. The regions of different types of solutions for the neuronal dynamics are depicted in the (quarter of the) plane of (non-negative) J21 and J12. (B). The limit cycle solution. The firing rate of populations 1 and 2 are plotted in black and blue, respectively, as a function of time (measured in units of τa) in the anti-phase oscillatory solution with T1 = 1.2 and T2 = 0.8, yielding J21 ≈ 2.36 and J12 ≈ 1.87 (see Eq. (43)). In this specific example we used I = 2, A = 2, the solid lines show the solution for and the dashed depict the solution in the limit of . (C) The oscillation period along the diagonal. The oscillation period on the diagonal is shown as a function of the reciprocal inhibition strength for different values of the adaptation strength, A = 0.25, 0.5, 1, 1.5 from left to right. Solid lines show the analytical relation of Eq. (44) in the limit. The circles depict the case. (D) The cross-correlation function. The neuronal cross-correlations Γ12 (green and black) and Γ21 (blue) are plotted as a function of the time difference, Δ (measured in units of the adaptation time constant τa). The black line depicts the correlations in the limit, whereas the green and blue lines show the case. Parameters were identical to B. For the case the correlations were evaluated from the numerical solution for the dynamics. (E) The ‘mean cross-correlation’ function. The mean correlation, Γ+, in the limit of , (see subsection Calculation of the cross-correlation function in Methods) is plotted as a function of Δ for T = 2 and different values of the T1 = T[0.1, 0.2, … 0.9] shown by color. Note that the plots for T1 = x and T1 = T − x overlap. (F) The ‘difference cross-correlation’. The difference in the cross-correlation, Γ−, in the limit of , is plotted as a function of Δ for T = 2 and different values of the T1 = T × {0.1, 0.2, … 0.9} shown by color from yellow (T1 = 0.1T) to blue (T1 = 0.9T). In E and F A = 2 and I = 2 were used.
For weak reciprocal inhibition, J21 < 1 + A and J12 < 1 + A, there is a solution in which both populations are active which we term the Fusion state. However, the Fusion state loses its stability for sufficiently strong inhibition, . Consequently, there is a region in the phase diagram in which there is no stable fixed point solution. In this region the system relaxes to a limit cycle (i.e., a periodic oscillatory solution, denoted by L.C. on the phase diagram) of anti-phase oscillation, Fig. 1B. In this case, the limit cycle solution has two phases. During phase 1, population 1 is dominant and active, r1 > 0, whereas population 2 is quiescent, r2 = 0. However, this state is not stable. Due to the adaptation, the activity of population 1 will decrease until population 2 is released from its inhibition and will further suppress population 1. During phase 2, population 2 is dominant and population 1 is quiescent. In the limit of slow adaptation, , a complete solution for the limit cycle can be derived; see the limit cycle solution section in Methods.
We denote by Ti the dominance time of population i, and by T = T1 + T2 the period of the oscillations; see Fig. 1B. Along the diagonal of the phase diagram, , the dominance times are equal, T1 = T2 = T/2, and the oscillation period monotonically increases from zero on the boundary of the stable Fusion solution, , to infinity on the boundary of the Rival solutions, , Fig. 1C. The dominance time of population 1, T1, diverges to infinity on the boundary of Rival 1 state, , and similarly T2, diverges on the boundary of Rival 2 state; see Eqs (40 and 43) in the limit cycle solution section in Methods. Thus, the basic features of the oscillatory solution can be understood from the geometry of the phase diagram.
The correlation function
One key factor that shapes STDP dynamics is the pre-post cross-correlation function. Because neuronal activities follow independent inhomogeneous Poisson processes statistics, the cross-correlation of different neurons is given by the product of their mean firing rates. Specifically, we are be interested in the temporal average of these correlations (see below). For a periodic solution we define
| 5 |
Figure 1D shows the temporal average cross-correlation, Γij(Δ), for finite (green and blue) and in the limit of in black. Note that the main difference is the slight deviation in the oscillation period due to finite , which is more important at low T. A detailed derivation of the cross-correlation functions appears in Methods. To analyze the STDP dynamics it is convenient to use the following quantities:
| 6 |
| 7 |
as shown in Fig. 1E,F, respectively, as a function of the time difference, Δ, for T = 2 and different values of T1 (differentiated by color). In general, Γ±(Δ) are periodic functions of the time difference, Δ, with a period of T. Γ+(Δ) is a positive even function of the time difference, Δ, that is symmetric with respect to T/2, whereas Γ−(Δ) is an odd function of Δ that is anti-symmetric with respect to T/2. Importantly, on the diagonal of the phase diagram, from symmetry, one obtains that Γ−(Δ) = 0.
The STDP rule
The above analysis was carried out for fixed values of the synaptic weights, assuming that the time scales in which the synaptic weights change are much longer than the characteristic times of the neuronal population dynamics, τm and τa (see e.g.24,29,32,36). Next we consider the effect of STDP. We assume that initially the synaptic weights are relatively weak (i.e., near the origin of the phase diagram in the Fusion state) and examine how activity dependent plasticity shapes its evolution. Hence, the STDP dynamics can be thought of as a flow on the phase diagram. We are interested in understanding how the features of the STDP rule shape this flow. In particular, we aim to elucidate when this flow leads the system into the limit cycle region. Following Luz and Shamir36 we write the STDP rule as the sum of two processes, potentiation and depression,
| 8 |
where ΔJ is the synaptic weight difference associated with pre and post spikes with a time difference of Δt = tpost − tpre. The functions K±(t) are the temporal kernels for the potentiation (+) and depression (−) of the STDP rule, respectively, and α is the relative strength of the depression. Parameter λ is the learning rate. We assume that the learning process occurs on a slower time scale than the adaptation. Specifically, here we focus on the family of temporally a-symmetric exponential learning rules:
| 9 |
where Θ(x) is the Heaviside step function, and τ± denote the characteristic time scales of the potentiation (+) and depression (−) branches of the rule. The parameter H = ±1 governs the nature of the learning rule, with H = 1 for a “Hebbian” rule (i.e., potentiating at the causal branch, when the post fires after pre, Δt > 0), and H = −1 for the “Anti-Hebbian” STDP rule. Below we analyze the mean field approximation in the limit of λ → 0.
STDP dynamics in the limit of slow learning
Deriving the dynamic equations
Changes to the synaptic weights following the plasticity rule of Eq. (8) in short time intervals occur as a result of either a pre or post-synaptic spike during this interval. Thus, we obtain
| 10 |
where is the spike train of the post/pre neuron written as a sum of delta function at the neuron’s spike times . In the limit of slow learning, λ → 0, the right hand side of Eq. (10) can be replaced by its temporal mean, yielding (see also24,29,32,36),
| 11 |
In regions of the phase diagram where a stable fixed point solution exists, i.e., , the correlation function is given by the product of the time independent means, , and one obtains that . As the firing rates are non-negative and the temporal kernels of the potentiation and depression, K±, have an integral of one, the sign of is determined by 1 − α. As a corollary, the synaptic weights will flow towards the region of the limit cycle solution from initial conditions close to the origin in the phase diagram if α < 1. This result holds for any choice of temporal structure for the STDP rule. In particular it is independent of the Hebbianity (the value of H) of the STDP rule. Note that a similar condition (α < 1) was assumed to be the biologically relevant choice for inhibitory plasticity in35. Thus, initial conditions of weak synaptic coefficients (Jij close to the origin) will flow towards the region of the limit cycle solution and will enter it near the diagonal, J21 = J12.
Order parameters of the STDP dynamics
In the region of the limit cycle the STDP dynamics do not necessarily flow in parallel to the identity line, but rather depend on the specific limit cycle solution and on the temporal structure of the STDP rule. It is convenient to formulate the STDP dynamics in terms of the mean and relative synaptic weights,
| 12 |
| 13 |
Using the above definitions, and averaging and subtracting Eq. (11) yields
| 14 |
For Γ± see Eqs (6 and 7) and Fig. 1E,F.
On the diagonal, J12 = J21, due to the symmetry of the limit cycle solution Γ12(t) = Γ21(t), and as a result . The mean correlation, Γ+, on the other hand, is a positive even function of time with a period of T. Near the boundary of stable Fusion, the oscillation frequency diverges, T → 0. In this limit (for ) the limit cycle solution for the neuronal responses will approach a square wave solution (with 50% duty cycle on J12 = J21) transitioning between 0 and 2I/(2 + A) in anti-phase. The mean correlation function, Γ+(Δ), will approach a triangular wave starting at 0 for Δ = 0 and peaking at 2I2/(2 + A)2 for Δ = T/2. Consequently, for T → 0, the integral on the right hand side of Eq. (14) will be dominated by the DC component of Γ+, yielding in this limit. Hence, the same condition that allows the STDP dynamics to enter the limit cycle region from the Fusion region will also cause it flow in the positive J+ direction after entering the Limit cycle region.
STDP dynamics along the diagonal
Equation (14) provides two non-linear equations for J+ and for J− that are coupled in a non trivial manner via the dependence of the correlations on the synaptic weights. However, on the diagonal of the phase diagram the situation is simplified: since the problem is reduced to a one dimensional flow. To analyze the dynamics of J+ on the diagonal it is convenient to write it as the sum of two terms:
| 15 |
| 16 |
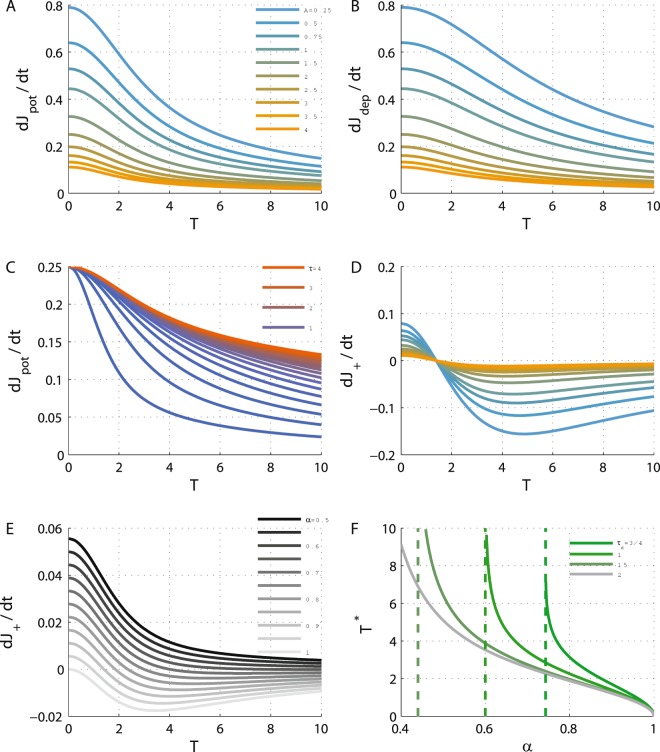
Figure 2A,B show and , respectively, on the diagonal as a function of the oscillation period, T (note that T is a function of , see e.g. Fig. 1C), for different values of A (differentiated by color). As can be seen from the figure, decreases monotonically from the value of I2/(2 + A)2 at T = 0 to 0 as T → ∞ at J12 = J21 = 1 + A (at the crossing to the bi-stable region). Due to the symmetry of the mean cross-correlation function, Γ+(t) = Γ+(−t), , and are independent of the Hebbianity of the STDP rule, H. Thus, the results of Fig. 2 hold for both Hebbian and Anti-Hebbian plasticity rules. Moreover, and only differ by the time constant of K±. Figure 2C shows as a function of the oscillation period, T, for different values of τ+ (depicted in color). All the curves decrease monotonically to zero, albeit with a different time scale; consequently, if τ+ < τ− then and there is equality only at T = 0 (on the boundary of stable Fusion).
Figure 2.
The dynamics of J+ along the diagonal. (A) The potentiation term, , of the mean synaptic weights, J+, Eq. (16), is shown as a function of the oscillation period along the diagonal for different values of A = 1/4, 1/2, 3/4, 1, 3/2, … 4 (from top at low A values to bottom). (B) The depression term, , of the mean synaptic weights, J+, Eq. (16), is shown as a function of the oscillation period along the diagonal for different values of the adaptation strength, A (as in A). (C) The effect of the STDP time constant. The potentiation term, , is shown as a function of the oscillation period along the diagonal for different values of τ+ = 1/4, 1/5, … 5, by different colors from blue (low τ+) to red. Here A = 2 was used. (D) The J+ dynamics along the diagonal. The value of is shown as a function of the oscillation period along the diagonal for different values of A using the same values and color code as in A, using α = 0.9. (E) The effect of the relative strength of depression. The value of is plotted as a function of the oscillation period along the diagonal for different values of α = 0.5, 0.55, … 1 from top (α = 0.5) to bottom (with A = 4). (F) Oscillation period at the STDP fixed point. The ‘learned’ oscillation period, T*, is shown as a function of α for different values of τ− differentiated by color. The vertical dashed lines depict the value of αc, see calculation of αc section in Methods. In all panels I = 2 was used, and λ = 1 was taken in D and E, for purposes of illustration. Unless otherwise stated, τ+ = 0.5 and τ− = 1 used. All units of time were measured in units of τa.
The dynamics of J+ along the diagonal are determined by the weighted sum of both and . will be positive for α < 1 for small T - near the crossing from the Fusion region. For τ+ < τ− and 1 > α > αc(τ+, τ−) (see Methods), will change its sign at T*; thus, the fixed point (note on the diagonal) at T* will be stable along the J+ direction. This scenario is illustrated in Fig. 2D that shows on the diagonal as a function of T (for different values of A, depicted by color). Interestingly, for this choice of exponential kernels for the STDP rule, the fixed point does not depend on the adaptation strength, A. The oscillation period at the fixed point, T*, is zero for α = 1 and diverges as α approaches a critical value αc(τ+, τ−), Fig. 2E,F, see subsection Calculation of αc in Methods. For fixed α ≤ 1 and τ+, T* is minimal for τ− → ∞, increases monotonically as τ− decreases and will diverge for a critical value τ−,c < τ+ such that αc(τ+, τ−) = α. For τ− < τ−,c (and α ≥ 1) there will be no fixed point along the diagonal and the STDP dynamics along the diagonal will flow outside of the limit cycle region.
STDP dynamics away from the diagonal
The stability of the STDP fixed point requires stability in the J− direction as well. On the diagonal . A small perturbation in the direction of J− will affect J− dynamics via the cross-correlation term Γ−(Δ), Eq. (14). The cross-correlations depend on the synaptic weight via the dominance times, T1 and T2. Hence, for a small perturbation around the diagonal, ΔJ− = J−, one obtains
| 17 |
The geometry of the phase diagram (Fig. 1A) reveals that increasing (decreasing) J− results in advancing towards the Rival 1 (Rival 2) region, and consequently increasing T1 (T2) and (decreasing) T− = T1 − T2; hence, .
As above, it is convenient to define
| 18 |
| 19 |
| 20 |
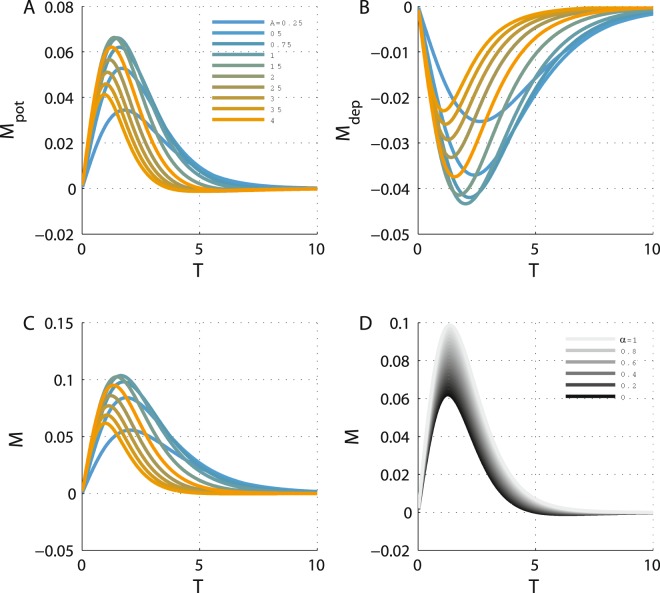
Similar to , is also written in the form of the integral of the product of two variables; namely, the learning rule and a term that depends on the cross-correlations. However, M+,pot/dep is not necessarily positive, as Γ−(Δ) and similarly are not necessarily positive. This is illustrated in Fig. 3A,B that show Mpot and Mdep, respectively, along the diagonal as a function of T for different values of A (depicted by color) for Hebbian STDP, H = 1. Moreover, Γ−(Δ) and similarly are odd functions of Δ. Consequently, and in Fig. 3A,B have different signs.
Figure 3.
Stability in the J− direction along the diagonal. (A) The value of Mpot is shown as a function of T along the diagonal of the phase diagram in the Limit cycle region for different values of A = 1/4, 1/2, 3/4, 1, 3/2, … 4 (from top at low A values to bottom). Here τ+ = 0.5 was used. All units of time were measured in units of τa. (B) The value of Mdep is shown as a function of T for different values of the adaptation strength, A (as in (A)). Here τ− = 1 was used. (C) J− dynamics along the diagonal. The value of M is shown as a function of the oscillation period along the diagonal for different values of A (same values and color code as in A), using α = 0.9. (D). The effect of the relative strength of depression. The value of M is plotted as a function of the oscillation period along the diagonal for different values of α = 0.5, 0.55, … 1 from bottom (dark, α = 0) to top (light, α = 1), using A = 2.
The value of M = Mpot − αMdep along the diagonal is depicted as a function of the oscillation period, T, for different values of A (differentiated by color) and α (shown by gray level) in Fig. 3C,D, respectively. Here, M is positive, and as a result, the STDP fixed point along the diagonal will be stable with respect to fluctuations in the J− direction for Hebbian plasticity.
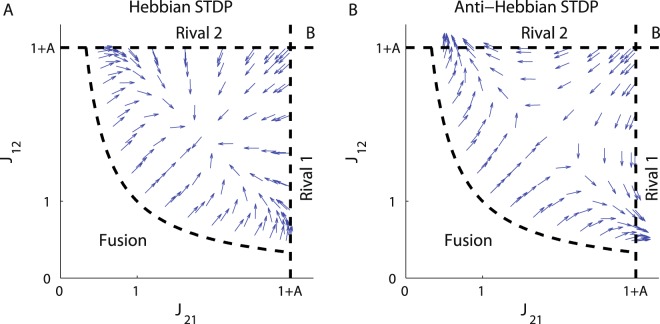
Finally, as Γ− and similarly are odd functions of time, switching from the Hebbian plasticity rule, H = 1, to Anti-Hebbian, H = −1, will result in a change of the sign of Mpot, Mdep and of M. Consequently, a fixed point (on the diagonal) that is stable in the J− direction for Hebbian plasticity will be unstable for Anti-Hebbian plasticity and vice versa. Figure 4 shows the flow induced by the STDP on the phase diagram for the (A) Hebbian and (B) Anti-Hebbian learning rules. As can be seen, the Anti-Hebbian learning rule is unable to converge to a state that allows oscillatory activity. In contrast, the Hebbian STDP generates symmetric (T1 = T2) anti-phase oscillatory activity in which the oscillation period is determined and controlled by the relative strength of the depression, α. This specific learning rule provides robustness with respect to the strength of adaptation, A. Fluctuations in A do not affect the period of the oscillation.
Figure 4.
The flow on the phase diagram. The direction of the dynamic flow; i.e., the normalized vector , is shown in the Limit cycle region of the phase diagram for (A). Hebbian plasticity, H = 1 in Eq. (9), and (B). Anti-Hebbian plasticity, H = −1. The parameters used here were: A = 2, τ+ = 0.5 and τ− = 1.
Discussion
We examined whether rhythmic activity can emerge via an unsupervised learning process of STDP. Our main result is that under a wide range of parameters, rhythmicity can develop via STDP. Specifically, we found that to develop the capacity for rhythmic activity, the STDP rule must obey the following conditions (i) a bias towards potentiation, α < 1, will lead the system into the oscillatory region of the phase diagram, (ii) a longer characteristic time for depression than for potentiation, τ− > τ+, will enable the existence of a fixed point on the diagonal that can be governed by the exact value of α, and (iii) the stability of the fixed point in the orthogonal direction is governed by the ‘Hebbianity’ of the plasticity rule.
Using the framework of a simplified toy-model, Magnasco and colleagues studied the computational implications of neuronal plasticity in recurrent networks80. It was claimed that Anti-Hebbian plasticity rules drive the network into a state of near criticality. This raises the question of why we did not find traces of criticality in our model. The explanation has to do with the differences between our models. The most significant is the temporal structure of the learning rule, which together with the neuronal cross-correlations is the driving force of STDP dynamics. In their work, Magnasco and colleagues used an instantaneous plasticity rule. Consequently, only correlations at a zero time difference, which are inherently symmetric, contributed to their learning dynamics. As a result, only the symmetric part of their synaptic connectivity pattern was affected by the dynamics. In contrast, in our model both J+ and J−, which denote the symmetric and anti-symmetric parts of the connectivity pattern - respectively, evolve with time. When one allows for a non-trivial STDP rule, much richer dynamical behaviors can develop60. Nevertheless, it is important to emphasize we do not claim that that Hebbian but not Anti-Hebbian plasticity will induce rhythmogenesis. We found that due to inherent symmetry if the Hebbian STDP fails to yield rhythmogenesis then the Anti-Hebbian can, and vice-versa.
Control of rhythmic activity
STDP may also provide a mechanism for selecting and stabilizing oscillations; for example, the oscillation frequency can be governed and manipulated by the relative strength of the depression, α, or changes in the time constants of the STDP rule, τ±, see Fig. 2F. Disruption of the STDP rule may result in changes to the learned oscillation frequency.
Simplifying assumptions
The analysis of STDP dynamics in recurrent networks is challenging. To facilitate the analysis we used the framework of a simplified model for the neuronal responses and made several simplifying assumptions. We assumed a separation of three time scales . The separation of the neuronal time constant from that of the adaptation enabled us to obtain an analytic expression for the temporal correlations that drive the STDP dynamics. The assumption that long term synaptic plasticity occurs on a longer time scale allowed us to consider STDP dynamics as a flow on the phase diagram. Numerous studies have employed phase diagram description to depict the possible dynamical states of the network as a function of various parameters. Our approach to STDP dynamics adds another layer to this description.
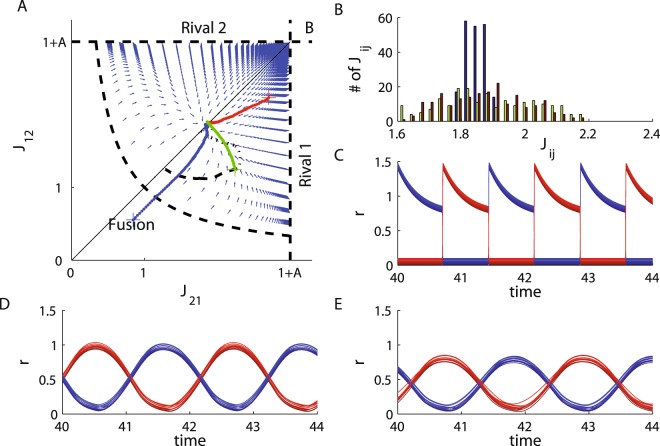
Figure 5 shows numerical solutions for the STDP dynamics. The vector field depicts our analytic solution to the STDP dynamics using exact expressions for the cross-correlations (see calculation of the cross-correlation function in Methods) in the limit of . The red, green and blue traces show the results of numerically simulating the STDP dynamic with a neuronal model with a small but finite . As can be seen from the figure, the numerical results with closely adhere to the analytically calculated flow and converge to the same fixed point. The dashed black line depicts the numerical results of simulating the model with . Taking affects the temporal pattern of the oscillations, Fig. 5D (compare with Fig. 5C for ); mainly no population is ever fully suppressed. This affects the cross-correlation function, which in turn will modify the flow along the phase diagram. As a result, the STDP dynamics will converge to a different fixed point. Nevertheless, the STDP dynamics still converges to a state of anti-phase oscillations, Fig. 5C. Thus, although quantitatively the results are different, similar qualitative behavior is obtained. The limit of small enabled us to obtain complete analytical expressions for the cross-correlations.
Figure 5.
Numerical simulation of STDP dynamics. We solved the STDP dynamics numerically, Eq. (11), with N1 = N2 = 10, α = 0.9, τ+ = 0.5 and τ− = 1. The cross-correlation functions were evaluated numerically using the separation of time scales. For each update step of the synaptic weights, the cross-correlations were evaluated by numerically solving the 2(N1 + N2) dynamics of the neuronal firing rates, Eqs (1–4), with fixed values for the synaptic weights with I = 2, A = 2 and . (A) Trajectories of the order parameters, , for five simulations are plotted on the phase diagram and the flow chart. The red, green and blue traces depict the learning dynamics of the same model with from different initial conditions (marked by +). The dashed black curve depicts the learning dynamics of the order parameters with . The dotted black curve depicts the learning dynamics of the order parameters for a model with a local inhibition term , and . The vector field shows the STDP flow for calculated using the analytic expressions for the correlations, subsection Calculation of the cross-correlation function in Methods. (B) Synaptic weight distribution for the three examples with in A (red, green and blue), differentiated by color. (C) Neuronal dynamics at the STDP fixed point for the slow adaptation case, . The firing rates of the N1 population 1 neurons (red traces) and N2 population 2 neurons (blue traces) in arbitrary units are shown as a function of time. Since the firing rates of different neurons from the same population overlapped, we shifted them vertically for purposes of visualization. For the three different initial conditions (with ) illustrated in A the oscillation period was T = 1.433, T = 1.432, and T = 1.436, and all units of time are measured in units of τa. (D) Neuronal dynamics with at the STDP fixed point. The firing rates of the N1 population 1 neurons (red traces) and N2 population 2 neurons (blue traces) in arbitrary units are shown as a function of time. The firing rates of different neurons were shifted vertically for purposes of visualization. The oscillation period was T = 2.165 in units of τa. (E) Neuronal dynamics with local inhibition, Jloc = 0.5, and at the STDP fixed point. The firing rates of the N1 population 1 neurons (red traces) and N2 population 2 neurons (blue traces) in arbitrary units are shown as a function of time, see subsection Neuronal dynamics with local inhibition term in Methods. The firing rates of different neurons were shifted vertically for purposes of visualization. The oscillation period was T = 2.17 in units of τa.
The interplay between short and long term plasticity processes deserves consideration. Oscillations would not be possible in this model without short term plasticity; here, adaptation. Thus, short term plasticity plays a major role in shaping the temporal structure of the neuronal cross-correlations, Γij(t) that drive the STDP dynamics, which in turn, may or may not converge to a state that allows this oscillatory behavior. It is interesting to note that short term plasticity, specifically the value of A, affects and shapes the phase diagram. Decreasing the value of A to zero, for example, will shrink the region of oscillatory activity to zero and rhythmic activity will no longer be possible.
The reflection of the flow on the phase diagram with respect to the diagonal when reflecting the STDP rule with respect to time stems from the inherent symmetry of the cross-correlation function which drives the dynamics (Γij(Δ) = Γji(−Δ)); hence, it is general and holds regardless of the choice of model. Certain other assumptions can easily be relaxed. For example, we assumed symmetry between the two competing populations. However, using the (threshold) linearity of our model one can easily rescale the neuronal responses to allow for different inputs and adaptation strengths. On the other hand, the independence of the fixed point, T*, on the adaptation strength, A, is specific to this model and for the choice of an exponentially decaying STDP rule.
Choice of network architecture
A central assumption in this study was the choice of (a reciprocal inhibition) architecture. Because it is not possible to analyze a system with an undefined architecture some choice had to be made. The specific choice of architecture was made to obtain a model that could be fully analyzed. However, the choice of architecture (including the short-term-plasticity mechanism) shapes the phase diagram, allows for the different regions of dynamical solutions (fixed points, In/Out of/Anti -phase oscillations, etc.) and determines the cross-correlations. Additionally, under certain conditions, propagation delays may also have a major effect on the computational outcome of the STDP dynamics81. For example, The dotted line in Fig. 5A shows the numerical results of simulating the STDP dynamics in a neuronal model that also incorporates within population inhibition, see section neuronal dynamics with local inhibition term in Methods. The STDP dynamics with local inhibition follows a different flow on the phase diagram and converges to a different point. Nevertheless, the STDP dynamics converges to a state of anti-phase oscillations, Fig. 5E. Incorporating a local inhibition term will not only modify the flow on the phase diagram, but will also change the phase diagram itself. Consequently, the effect of the network architecture on STDP dynamics should not be underestimated. Because this effect is highly non-linear, one cannot generalize these results to other architectures in a straightforward manner. Nevertheless, the approach delineated here; namely, studying the induced flow on the phase diagram of the system, can be applied to other models in the limit of a slow learning rate.
Robustness to synaptic variability
Yet another central simplifying assumption we made throughout our analysis was that the population-mean firing rates and the mean synaptic weights were representative of single neuron firing rates and single synaptic weights. This allowed us to explore a model with reduced dimensionality (the phase diagram was analyzed in 2 dimensions instead of 2N1N2 dimensions) and only to study the dynamics of the global order parameters such as the effective (or mean) couplings between the two populations. However, it is not at all obvious that the mean synaptic weight is indeed a good representative of the synaptic weight distribution, since for instance, the neuronal populations may break down into sub-clusters. Figure 5A shows that even when individual synapses are free to potentiate and depress independently, the mean weights follow the predicted flow on the phase diagram. Moreover, the mean synaptic weights remain representative of the individual weights that are distributed around them, Fig. 5B. Nevertheless, the distribution around the mean weights is not trivial. Consequently, different neurons may receive different levels of inhibition and miss-tuning of the oscillation frequency might occur. Figure 5C shows that the firing rates of different neurons (vertically shifted) in the two populations (differentiated by color) are identical in spite of the synaptic weights distribution. Moreover, even though synaptic weight distribution is different in the three examples shown (differentiated by color), the oscillation period is almost identical. Thus, functionality, in terms of obtaining a specific oscillation frequency, is retained even in the face of synaptic variability. What is the source of this remarkable outcome? We believe that this results from the fact that the STDP dynamics (e.g. Eq. (11)) only depend on the synaptic weights via the cross-correlations, which in turn, are determined by the oscillation period and dominance times. Thus, the fixed point of the STDP dynamics itself is determined by the oscillation period due to the activity dependence of the plasticity rule. On the other hand, to obtain this rhythmic activity it was also essential to have an architecture with two distinct inhibitory populations.
Methods
Phase diagram and limit cycle calculations
The fixed points of the dynamics
From Eqs (1–4) we obtain the dynamics of the mean firing rates in each population
| 21 |
| 22 |
| 23 |
| 24 |
We also rescaled time and hereafter measure time in units of the adaptation time constant. We distinguish two types of fixed points: Rival states, in which one population fully suppresses the other, and Fusion, in which both populations are active.
The Rival states
The Rival-1 solution assumes and , yielding , and . The existence condition for this solution is that the net input to population 2, I − J21r1 − a2 is non-positive, at the fixed point, J21 ≥ 1 + A. This solution is always stable where it exists.
The Fusion state
The Fusion solution assumes and , yielding
| 25 |
| 26 |
where . The existence of the Fusion solution requires the inputs of both populations to be non-negative. For the existence condition requires J12 ≤ 1 + A and J21 ≤ 1 + A (bottom left square in the phase diagram, Fig. 1A, where no Rival solution exists). By contrast, for the existence condition requires J12 ≥ 1 + A and J21 ≥ 1 + A (the region in the phase diagram where both Rival solutions exist). However, the Fusion state is not always stable. By performing standard stability analysis around the Fusion fixed point we expand the dynamics around the fixed point to a leading order in the fluctuations
| 27 |
where , yielding the four eigenvalues for the stability matrix:
| 28 |
The sum of the pair of eigenvalues is and their product is ; hence, these eigenvalues are always stable. On the other hand, for the pair of eigenvalues the sum is , which is negative if and only if inhibition is sufficiently weak, (in that case their product will also be positive, assuming is small). Thus, the Fusion state loses its stability when reciprocal inhibition becomes sufficiently strong, .
The limit cycle solution
In the region of the phase diagram where no stable fixed point exists the network dynamics relaxes to anti-phase oscillations. Below we provide a detailed solution for the limit cycle in the limit of . The limit cycle is solved using the anti-phase oscillations ansatz. First the neuronal dynamics is solved for each phase, where the dynamics are linear. This provides a piecewise solution with several parameters to be determined. Then we apply two sets of constraints: periodicity and transition.
Assuming the anti-phase oscillations ansatz we separate the cycle into two phases. During phase-1 population 1 is dominant and fully suppresses population 2, for times t ∈ (0, T1). In the limit of slow adaptation, , dynamics during phase-1 are given by:
| 29 |
| 30 |
| 31 |
| 32 |
where we measure time in units of τa. Eqs (29–32) can be easily solved, yielding
| 33 |
| 34 |
Similarly, during phase-2, when population 2 is dominant and fully suppresses population 1, , we obtain
| 35 |
| 36 |
Continuity of the adaptation variables, ai, dictates that, for example, the initial conditions of Eq. (36), a2(T1), will be given from Eq. (34) by . We now need to determine four parameters: a1(0), a2(0), T1 and T2. These parameters are determined by two sets of constraints. One is periodicity, namely
| 37 |
yielding,
| 38 |
| 39 |
| 40 |
The second set of constraints is given by the transition conditions. Specifically, the transition time from phase-1 to phase-2 at T1 is not arbitrary; rather, T1 is a special point in time in which population 2 is released from being fully suppressed, such that the net input to population 2 changes its sign from negative to positive; thus,
| 41 |
| 42 |
which provides implicit equations for the dominance times, T1 and T2,
| 43 |
Using Eq. (43), and taking the limit of T1 → ∞, we obtain J21 → 1 + A. Thus, the dominance time of population i, Ti, diverges on the boundary of Rival-i. Taking the limit of T1, T2 → 0 such that T1/T2 = β, yields and from symmetry , which obeys J12J21 → 1; hence, the limit of the zero oscillation period is obtained on the boundary of stable Fusion (note that these calculations were done for ).
On the diagonal, , dominance times are equal, T1 = T2 = T/2,
| 44 |
Consequently, the oscillation period, T, increases monotonically along the diagonal of the phase-diagram from zero at the transition to Fusion () to infinity at the transition to the Rival states ().
Calculation of the cross-correlation function
Calculation of the (temporally averaged) cross-correlation function, Eq. (5), is done using the analytical solution for the neuronal responses in the limit of slow adaptation, . These correlations arise from co-fluctuation of the firing rates of the neurons and affect the STDP dynamics via their overlap with the STDP rule; thus, the relevant timescales are determined by the temporal structure of the STDP rule, Eq. (9). When the system relaxes to a fixed point solution, (i = 1, 2), the cross-correlations are constant in time,
| 45 |
Thus, correlations will be zero in the Rival states; hence, there will be no STDP. In the Fusion state the cross-correlations will be symmetric, Γ12(t) = Γ21(t). As a result, the STDP dynamics for J12 and J21 will be identical and the flow will be in the uniform direction, parallel to the diagonal line.
At the Limit cycle we use the analytical solution, Eqs (33–40), to calculate the cross-correlations in a straightforward manner. For we obtain
| 46 |
| 47 |
| 48 |
| 49 |
| 50 |
where
| 51 |
For Δ > min{T1, T2}, assuming without loss of generality that T1 ≥ T2
| 52 |
| 53 |
| 54 |
| 55 |
| 56 |
Along the diagonal, on the edge of the stable Fusion state region, T → 0, the cross-correlation will resemble a triangular chainsaw function (in the limit) with period T and peak 2I2/(2 + A)2. Consequently, as T goes to zero, the overlap between the cross-correlation function and the STDP rule will be governed by the DC component, yielding
| 57 |
The above expressions for the cross-correlations were given in terms of the dominance times, {Ti} instead of the effective couplings Jij. The translation to the synaptic weights from the dominance times is possible by Eq. (43). However, because we were interested in studying the ability to learn and stabilize a specific oscillatory activity, it was more convenient to think about the dynamics in terms of the dominance times. Similarly, to consider stability with respect to the J− direction we utilized the derivative of Γ− = Γ21 − Γ12 with respect to T− = T1 − T2. On the diagonal,
| 58 |
| 59 |
| 60 |
| 61 |
| 62 |
Calculation of αc
On the diagonal T1 = T2 = T/2, in the limit of slow oscillations, T → ∞, one obtains
| 63 |
Using Eq. (63) yields
| 64 |
| 65 |
Hence, if α is less than a critical value αc = N(τ+)/N(τ−), then will always be positive (along the diagonal). On the other hand, if α is larger than αc then will be negative for sufficiently large T, and a fixed point will exist if α < 1.
Neuronal dynamics with a local inhibition term
In Fig. 5 we also show results of simulating the STDP dynamics in a model that includes a local, within population, inhibition. To this end we replace Eqs (1–4) with
| 66 |
| 67 |
| 68 |
| 69 |
Acknowledgements
This research was supported by THE ISRAEL SCIENCE FOUNDATION (grant No. 300/16). The firing rate model of two populations with reciprocal inhibition and slow adaptation was developed by H. Sompolinsky to model binocular rivalry.
Author Contributions
M.S. conceived the project, S.S. and M.S. carried out the analyses, M.S. wrote the main manuscript text, all authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buzsaki, G. Rhythms Of The Brain (Oxford University Press, 2006).
- 2.Buzsaki G, Freeman W. Editorial overview: brain rhythms and dynamic coordination. Curr. Opin Neurobiol. 2015;31:v–ix. doi: 10.1016/j.conb.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Bocchio M, Nabavi S, Capogna M. Synaptic Plasticity, Engrams, and Network Oscillations in Amygdala Circuits for Storage and Retrieval of Emotional Memories. Neuron. 2017;94:731–743. doi: 10.1016/j.neuron.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Shamir M, Ghitza O, Epstein S, Kopell N. Representation of time-varying stimuli by a network exhibiting oscillations on a faster time scale. PLoS Comput. Biol. 2009;5:e1000370. doi: 10.1371/journal.pcbi.1000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taub AH, Perets R, Kahana E, Paz R. Oscillations synchronize Amygdala-to-prefrontal primate circuits during aversive learning. Neuron. 2018;97:291–298e3. doi: 10.1016/j.neuron.2017.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Cao W, et al. Gamma oscillation dysfunction in mPFC leads to social deficits in neuroligin 3 R451C knockin mice. Neuron. 2018;97:1253–1260e1257. doi: 10.1016/j.neuron.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh S, Laxmi TR, Chattarji S. Functional connectivity from the amygdala to the hippocampus grows stronger after stress. J. Neurosci. 2013;33:7234–7244. doi: 10.1523/JNEUROSCI.0638-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Andreae LC, Burrone J. The role of neuronal activity and transmitter release on synapse formation. Curr. Opin. Neurobiol. 2014;27:47–52. doi: 10.1016/j.conb.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verhage M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 11.Hebb, D. O. The Organization Of Behavior; A Neuropsychological Theory (Wiley, 1949).
- 12.Bi G, Poo M. Synaptic modification by correlated activity: Hebb’s postulate revisited. Annu. Rev. Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- 13.Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- 14.Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387:278–281. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- 15.Woodin MA, Ganguly K, Poo MM. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl- transporter activity. Neuron. 2003;39:807–820. doi: 10.1016/S0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]
- 16.Haas JS, Nowotny T, Abarbanel HD. Spike-timing-dependent plasticity of inhibitory synapses in the entorhinal cortex. J. Neurophysiol. 2006;96:3305–3313. doi: 10.1152/jn.00551.2006. [DOI] [PubMed] [Google Scholar]
- 17.Vogels TP, et al. Inhibitory synaptic plasticity: spike timing-dependence and putative network function. Front. Neural Circuits. 2013;7:119. doi: 10.3389/fncir.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- 20.Zhang LI, Tao HW, Holt CE, Harris WA, Poo MM. A critical window for cooperation and competition among developing retinotectal synapses. Nature. 1998;395:37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]
- 21.Dan Y, Poo MM. Spike timing-dependent plasticity: From synapse to perception. Physiological Reviews. 2006;86:1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- 22.Zilberter M, et al. Input Specificity and Dependence of Spike Timing-Dependent Plasticity on Preceding Postsynaptic Activity at Unitary Connections between Neocortical Layer 2/3 Pyramidal Cells. Cerebral Cortex. 2009;19:2308–2320. doi: 10.1093/cercor/bhn247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cateau H, Fukai T. A stochastic method to predict the consequence of arbitrary forms of spike-timing-dependent plasticity. Neural Comput. 2003;15:597–620. doi: 10.1162/089976603321192095. [DOI] [PubMed] [Google Scholar]
- 24.Gütig R, Aharonov R, Rotter S, Sompolinsky H. Learning input correlations through nonlinear temporally asymmetric Hebbian plasticity. J. Neurosci. 2003;23:3697–3714. doi: 10.1523/JNEUROSCI.23-09-03697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison A, Diesmann M, Gerstner W. Phenomenological models of synaptic plasticity based on spike timing. Biol. Cybern. 2008;98:459–478. doi: 10.1007/s00422-008-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin J, Lee DD, Sompolinsky H. Equilibrium properties of temporally asymmetric Hebbian plasticity. Phys. Rev. Lett. 2001;86:364–367. doi: 10.1103/PhysRevLett.86.364. [DOI] [PubMed] [Google Scholar]
- 27.Song S, Abbott LF. Cortical development and remapping through spike timing-dependent plasticity. Neuron. 2001;32:339–350. doi: 10.1016/S0896-6273(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 28.Song S, Miller KD, Abbott LF. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat. Neurosci. 2000;3:919–926. doi: 10.1038/78829. [DOI] [PubMed] [Google Scholar]
- 29.Kempter R, Gerstner W, von Hemmen JL. Hebbian learning and spiking neurons. Physical Review E. 1999;59:4498–4514. doi: 10.1103/PhysRevE.59.4498. [DOI] [Google Scholar]
- 30.Gilson M, Burkitt AN, Grayden DB, Thomas DA, van Hemmen JL. Emergence of network structure due to spike-timing-dependent plasticity in recurrent neuronal networks III: Partially connected neurons driven by spontaneous activity. Biol. Cybern. 2009;101:411–426. doi: 10.1007/s00422-009-0343-4. [DOI] [PubMed] [Google Scholar]
- 31.Gilson M, Burkitt AN, Grayden DB, Thomas DA, van Hemmen JL. Emergence of network structure due to spike-timing-dependent plasticity in recurrent neuronal networks IV - Structuring synaptic pathways among recurrent connections. Biol. Cybern. 2009;101:427–444. doi: 10.1007/s00422-009-0346-1. [DOI] [PubMed] [Google Scholar]
- 32.Gilson M, Burkitt AN, Grayden DB, Thomas DA, van Hemmen JL. Emergence of network structure due to spike-timing-dependent plasticity in recurrent neuronal networks. I. Input selectivity-strengthening correlated input pathways. Biol. Cybern. 2009;101:81–102. doi: 10.1007/s00422-009-0319-4. [DOI] [PubMed] [Google Scholar]
- 33.Gilson M, Burkitt AN, Grayden DB, Thomas DA, van Hemmen JL. Emergence of network structure due to spike-timing-dependent plasticity in recurrent neuronal networks. II. Input selectivity-symmetry breaking. Biol. Cybern. 2009;101:103–114. doi: 10.1007/s00422-009-0320-y. [DOI] [PubMed] [Google Scholar]
- 34.Gilson M, Burkitt AN, Grayden DB, Thomas DA, van Hemmen JL. Emergence of network structure due to spike-timing-dependent plasticity in recurrent neuronal networks V: self-organization schemes and weight dependence. Biol. Cybern. 2010;103:365–386. doi: 10.1007/s00422-010-0405-7. [DOI] [PubMed] [Google Scholar]
- 35.Luz Y, Shamir M. Balancing feed-forward excitation and inhibition via Hebbian inhibitory synaptic plasticity. PLoS Comput. Biol. 2012;8:e1002334. doi: 10.1371/journal.pcbi.1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luz Y, Shamir M. The effect of STDP temporal kernel structure on the learning dynamics of single excitatory and inhibitory synapses. PLoS One. 2014;9:e101109. doi: 10.1371/journal.pone.0101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat. Neurosci. 2000;3:1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- 38.Kistler WM, van Hemmen JL. Modeling synaptic plasticity in conjuction with the timing of pre- and postsynaptic action potentials. Neural Comput. 2000;12:385–405. doi: 10.1162/089976600300015844. [DOI] [PubMed] [Google Scholar]
- 39.Vogels TP, Sprekeler H, Zenke F, Clopath C, Gerstner W. Inhibitory Plasticity Balances Excitation and Inhibition in Sensory Pathways and Memory Networks. Science. 2011;334:1569–1573. doi: 10.1126/science.1211095. [DOI] [PubMed] [Google Scholar]
- 40.Maffei A. Fifty shades of inhibition. Curr. Opin. Neurobiol. 2017;43:43–47. doi: 10.1016/j.conb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravid Tannenbaum N, Burak Y. Shaping Neural Circuits by High Order Synaptic Interactions. PLoS Comput. Biol. 2016;12:e1005056. doi: 10.1371/journal.pcbi.1005056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett JE, Bair W. Refinement and Pattern Formation in Neural Circuits by the Interaction of Traveling Waves with Spike-Timing Dependent Plasticity. PLoS Comput. Biol. 2015;11:e1004422. doi: 10.1371/journal.pcbi.1004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honda M, Urakubo H, Tanaka K, Kuroda S. Analysis of Development of Direction Selectivity in Retinotectum by a Neural Circuit Model with Spike Timing-Dependent Plasticity. Journal of Neuroscience. 2011;31:1516–1527. doi: 10.1523/JNEUROSCI.3811-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller KD, Mackay DJC. The Role of Constraints in Hebbian Learning. Neural Computation. 1994;6:100–126. doi: 10.1162/neco.1994.6.1.100. [DOI] [Google Scholar]
- 45.Senn W, Buchs NJ. Spike-based synaptic plasticity and the emergence of direction selective simple cells: Mathematical analysis. Journal of Computational Neuroscience. 2003;14:119–138. doi: 10.1023/A:1021935100586. [DOI] [PubMed] [Google Scholar]
- 46.Wenisch OG, Noll J, van Hemmen JL. Spontaneously emerging direction selectivity maps in visual cortex through STDP. Biological Cybernetics. 2005;93:239–247. doi: 10.1007/s00422-005-0006-z. [DOI] [PubMed] [Google Scholar]
- 47.Hennequin G, Agnes EJ, Vogels TP. Inhibitory Plasticity: Balance, Control, and Codependence. Annu. Rev. Neurosci. 2017;40:557–579. doi: 10.1146/annurev-neuro-072116-031005. [DOI] [PubMed] [Google Scholar]
- 48.Sprekeler H. Functional consequences of inhibitory plasticity: homeostasis, the excitation-inhibition balance and beyond. Curr. Opin. Neurobiol. 2017;43:198–203. doi: 10.1016/j.conb.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012;490:219–225. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ackman JB, Crair MC. Role of emergent neural activity in visual map development. Curr. Opin. Neurobiol. 2014;24:166–175. doi: 10.1016/j.conb.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cateau H, Kitano K, Fukai T. Interplay between a phase response curve and spike-timing-dependent plasticity leading to wireless clustering. Phys. Rev. E. 2008;77:051909. doi: 10.1103/PhysRevE.77.051909. [DOI] [PubMed] [Google Scholar]
- 52.Gerstner W, Kempter R, van Hemmen JL, Wagner H. A neuronal learning rule for sub-millisecond temporal coding. Nature. 1996;383:76–81. doi: 10.1038/383076a0. [DOI] [PubMed] [Google Scholar]
- 53.Gilson M, Burck M, Burkitt AN, van Hemmen JL. Frequency selectivity emerging from spike-timing-dependent plasticity. Neural Comput. 2012;24:2251–2279. doi: 10.1162/NECO_a_00331. [DOI] [PubMed] [Google Scholar]
- 54.Karbowski J, Ermentrout GB. Synchrony arising from a balanced synaptic plasticity in a network of heterogeneous neural oscillators. Phys. Rev. E. 2002;65:031902. doi: 10.1103/PhysRevE.65.031902. [DOI] [PubMed] [Google Scholar]
- 55.Kerr RR, Burkitt AN, Thomas DA, Gilson M, Grayden DB. Delay selection by spike-timing-dependent plasticity in recurrent networks of spiking neurons receiving oscillatory inputs. PLoS Comput. Biol. 2013;9:e1002897. doi: 10.1371/journal.pcbi.1002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S, Sen K, Kopell N. Cortical gamma rhythms modulate NMDAR-mediated spike timing dependent plasticity in a biophysical model. PLoS Comput. Biol. 2009;5:e1000602. doi: 10.1371/journal.pcbi.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masquelier T, Hugues E, Deco G, Thorpe SJ. Oscillations, phase-of-firing coding, and spike timing-dependent plasticity: an efficient learning scheme. J. Neurosci. 2009;29:13484–13493. doi: 10.1523/JNEUROSCI.2207-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muller L, Brette R, Gutkin B. Spike-timing dependent plasticity and feed-forward input oscillations produce precise and invariant spike phase-locking. Front. Comput. Neurosci. 2011;5:45. doi: 10.3389/fncom.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfister JP, Tass PA. STDP in Oscillatory Recurrent Networks: Theoretical Conditions for Desynchronization and Applications to Deep Brain Stimulation. Front. Comput. Neurosci. 2010;4:22. doi: 10.3389/fncom.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luz Y, Shamir M. Oscillations via Spike-Timing Dependent Plasticity in a Feed-Forward Model. PLoS Comput. Biol. 2016;12:e1004878. doi: 10.1371/journal.pcbi.1004878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ratnadurai-Giridharan S, Khargonekar PP, Talathi SS. Emergent gamma synchrony in all-to-all interneuronal networks. Front. Comput. Neurosci. 2015;9:127. doi: 10.3389/fncom.2015.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bayati M, Valizadeh A, Abbassian A, Cheng S. Self-organization of synchronous activity propagation in neuronal networks driven by local excitation. Front. Comput. Neurosci. 2015;9:69. doi: 10.3389/fncom.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Izhikevich EM, Edelman GM. Large-scale model of mammalian thalamocortical systems. Proc. Natl. Acad. Sci. USA. 2008;105:3593–3598. doi: 10.1073/pnas.0712231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin DHZ, Seung S. Fast computation with spikes in a recurrent neural network. Physical Review E. 2002;65:051922. doi: 10.1103/PhysRevE.65.051922. [DOI] [PubMed] [Google Scholar]
- 65.Fukai T, Tanaka S. A simple neural network exhibiting selective activation of neuronal ensembles: From winner-take-all to winners-share-all. Neural Computation. 1997;9:77–97. doi: 10.1162/neco.1997.9.1.77. [DOI] [PubMed] [Google Scholar]
- 66.Shamir M. The scaling of winner-takes-all accuracy with population size. Neural Comput. 2006;18:2719–2729. doi: 10.1162/neco.2006.18.11.2719. [DOI] [PubMed] [Google Scholar]
- 67.Hertz, J., Krogh, A. & Palmer, R. G. Introduction To The Theory Of Neural Computation. (Addison Wesley Publishing Company, 1991).
- 68.Bazhenov M, Huerta R, Smith BH. A computational framework for understanding decision making through integration of basic learning rules. J. Neurosci. 2013;33:5686–5697. doi: 10.1523/JNEUROSCI.4145-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Machens CK, Romo R, Brody CD. Flexible control of mutual inhibition: a neural model of two-interval discrimination. Science. 2005;307:1121–1124. doi: 10.1126/science.1104171. [DOI] [PubMed] [Google Scholar]
- 70.Aksay E, et al. Functional dissection of circuitry in a neural integrator. Nat. Neurosci. 2007;10:494–504. doi: 10.1038/nn1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci. 2016;19:1636–1646. doi: 10.1038/nn.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koyama M, Pujala A. Mutual inhibition of lateral inhibition: a network motif for an elementary computation in the brain. Curr. Opin. Neurobiol. 2018;49:69–74. doi: 10.1016/j.conb.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 73.Zohar O, Shamir M. A Readout Mechanism for Latency Codes. Front. Comput. Neurosci. 2016;10:107. doi: 10.3389/fncom.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hansel, D. & Sompolinsky, H. Modeling feature selectivity in local cortical circuits. In Methods in neuronal modeling: From synapses to networks (eds Koch, C. & Segev, I.) 499–567 (MIT Press, 1998).
- 75.Brette R, Gerstner W. Adaptive exponential integrate-and-fire model as an effective description of neuronal activity. J. Neurophysiol. 2005;94:3637–3642. doi: 10.1152/jn.00686.2005. [DOI] [PubMed] [Google Scholar]
- 76.Matsuoka K. The Dynamic-Model of Binocular-Rivalry. Biol. Cybern. 1984;49:201–208. doi: 10.1007/BF00334466. [DOI] [PubMed] [Google Scholar]
- 77.Li HH, Rankin J, Rinzel J, Carrasco M, Heeger DJ. Attention model of binocular rivalry. Proc. Natl. Acad. Sci. USA. 2017;114:E6192–E6201. doi: 10.1073/pnas.1620475114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Friesen WO. Reciprocal inhibition: a mechanism underlying oscillatory animal movements. Neurosci. Biobehav. Rev. 1994;18:547–553. doi: 10.1016/0149-7634(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 79.Shriki O, Hansel D, Sompolinsky H. Rate models for conductance-based cortical neuronal networks. Neural Comput. 2003;15:1809–1841. doi: 10.1162/08997660360675053. [DOI] [PubMed] [Google Scholar]
- 80.Magnasco MO, Piro O, Cecchi GA. Self-tuned critical anti-Hebbian networks. Phys. Rev. Lett. 2009;102:258102. doi: 10.1103/PhysRevLett.102.258102. [DOI] [PubMed] [Google Scholar]
- 81.Madadi Asl M, Valizadeh A, Tass PA. Dendritic and Axonal Propagation Delays Determine Emergent Structures of Neuronal Networks with Plastic Synapses. Sci. Rep. 2017;7:39682. doi: 10.1038/srep39682. [DOI] [PMC free article] [PubMed] [Google Scholar]