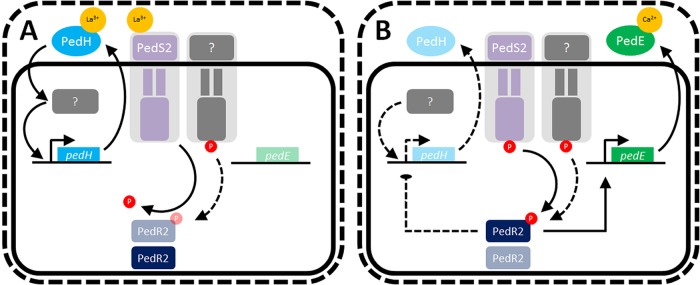
FIG 5 .
Working hypothesis of rare earth element (REE)-mediated switch of pedE and pedH in Pseudomonas putida KT2440. (A) The presence of REEs in the medium leads to binding of Ln3+ ions to PedH and the periplasmic domain of PedS2. The binding to PedH leads to the catalytic activation of the enzyme and triggers enhanced transcriptional activation of pedH by a so-far unknown mechanism and regulator, which is indicated by a gray box (8). Binding to the periplasmic domain of PedS2 leads to an outside-in signaling via the HAMP domain and decreases the kinase activity of the protein toward its cognate response regulator PedR2. In addition, this state of the PedS2 sensor is believed to exhibit phosphatase activity to reduce cross talk of an unidentified kinase (indicated by a gray membrane-bound protein) with activity toward PedR2 (for a more detailed explanation, see the text). (B) In the absence of REEs, the sensor kinase PedS2 is active and phosphorylates the cognate response regulator PedR2 at position D53. The phosphorylated PedR2 has a dual regulatory function as a strong pedE activator and a repressor of pedH. Black lines indicate known regulations or functionalities. Solid lines indicate a strong regulatory effect on genes or production of enzymes, whereas dotted lines indicate weaker regulatory effects or low production of enzymes.