Abstract
Pancreatic cancer is typically arises in the context of inflammation, and a surrounding area of pancreatitis is often present within the tumor microenvironment. Signet ring cell carcinoma (SRCC) is a rare variant of pancreatic adenocarcinoma. Pathologically, it presents either as single cells or loose clusters masquerading in the background of pancreatitis. Sampling of these inflammatory cells during biopsy can result in the incorrect diagnosis of pancreatitis. We report a case of SRCC of the pancreas which the diagnosis of cancer was delayed because multiple biopsies revealed only inflammatory changes with no obvious evidence of malignancy. This case highlights the fact that negative results with endoscopic ultrasound fine needle aspiration in SRCC can be misleading. A cancer diagnosis should still be considered despite findings of inflammatory pancreatitis if the clinical presentation is concerning for cancer (mass on CT scan).
INTRODUCTION
Signet ring cell carcinoma (SRCC) is extremely rare and occur in <1% of pancreatic cancers with only a few cases reported in the literature (Table 1). More than 96% of all SRCC arise in the stomach [1], however, cases occurring in the breast, gallbladder and colon have been reported. SRCC is characterized by large intra-cytoplasmic mucin vacuoles that expand in the malignant cells pushing the nucleus of the cell to periphery, creating a ‘signet ring’ configuration. When ≥50% of the tumor is composed of cells of this type, it is classified as a SRCC [2].
Table 1.
Summary of case reports of signet ring cell carcinoma of pancreas.
| Case | Presenting features | Autopsy/EUS findings |
|---|---|---|
| 1. Mc Arthur | 69 F with diffuse mass in head of pancreas and ampulla of Vater | SRCC Dx by EUS, extending into ampulla of Vater |
| 2. Tracey | 69 M diffuse mass with distal common bile duct obstruction | SRCC Dx at autopsy, obstructing common bile duct (CBD) |
| 3. Terada | 61 M presented with abd pain | SRCC Dx ERCP with tumor penetrating lumen of pancreatic duct |
| 4. Karaahmet | 83 M mass in pancreatic head and distal colon | SRCC Dx by EUS |
| 5. Nauta | 71 M mass in pancreatic head with CBD dilation | Negative ERCP with autopsy revealing SRCC |
| 6. Radojkovic | 67 F mass in pancreatic head | Tru-cut needle biopsy revealing SRCC |
| 7. Chow | 88 M dilated intra-hepatic duct | SRCC Dx by autopsy |
In the case of pancreatic cancer, inflammation is both as a risk factor, also a consequence of the cancer [3]. Additionally, pancreatitis an independent risk factor for pancreatic carcinoma, occasionally present with a mass or mass-like process. Pathologically, the signet-ring cells are seen floating in abundant extracellular mucin pools either as single cells or in loose clusters having loose contact with the surrounding structure
SRCC has a poor prognosis, and early diagnosis is of importance to the physician as well to the patient. Though endoscopic ultrasound fine needle aspiration (EUS-FNA) provides high diagnostic accuracy in most settings, one limitation related to this technique is that it often only provides a cytologic specimen with scant cellularity and repeated biopsies for accurate diagnosis increases risk of seeding malignant cells. The EUS tru-cut biopsy needle design can overcome the shortcomings of EUS-FNA by acquiring larger tissue samples allowing histologic examination [4].
CASE PRESENTATION
A 62-year-old female with history of Crohn’s was referred to our hospital with abdominal pain that started 5 months ago accompanied by nausea and vomiting. Review of systems was negative for weight loss or loss of appetite. She had no previous history of pancreatitis or family history of pancreatic cancer. The patient’s complete blood count and comprehensive metabolic panel were within normal limits. Her Ig G and ANCA levels were normal, thus ruling out autoimmune pancreatitis. CT revealed a diffuse tissue mass in the head and tail of the pancreas (Fig. 2). Fine needle aspiration of the mass through endoscopic ultrasound yielded only chronic inflammatory cells (lymphocytes, lymphocytic aggregates, macrophages, lympho histiocytic aggregates). A CT-guided percutaneous FNA of a peripancreatic lymph node also revealed no evidence of malignancy.
Figure 2:
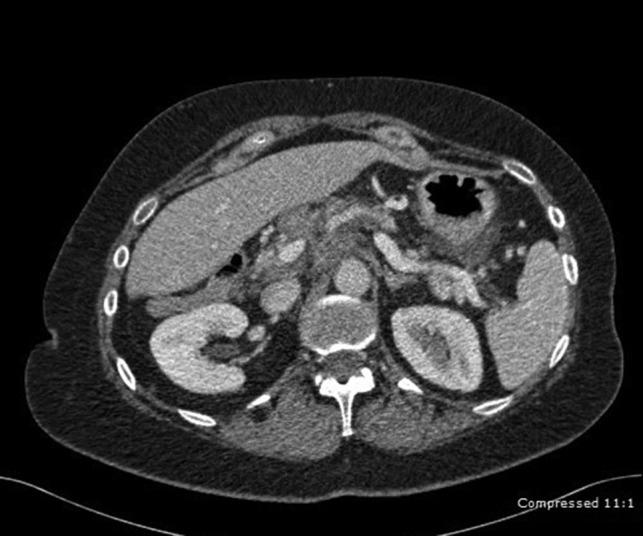
CT abdomen depicting a diffuse tissue mass in the head and tail of the pancreas.
Over a duration of 4 weeks, the patient lost ~10 pounds, and serum CA 19-9 increased from 73.5 to 405. Due to the concern for a malignant mass, patient underwent an exploratory laparotomy in order to obtain a definitive tissue diagnosis. During the operation, FNA biopsy was done of the mass. This was found to be suspicious for cancer. A shave biopsy was also done revealing adenocarcinoma. On further evaluation, the mass was found to be unresectable due to encasement of the superior mesenteric artery. Histopathological sections showed single and loose clusters of malignant cells with poor gland formation and occasional signet ring cells scattered within a desmoplastic and fibrotic stroma with some associated background mucin (Fig 1 a–c). Chronic pancreatitis-like changes were seen adjacent and admixed with the tumor cells (Fig. 1d). The final diagnosis was pancreatic poorly differentiated adenocarcinoma with signet ring cell features. The patient refused treatment and expired in 8 weeks following diagnosis.
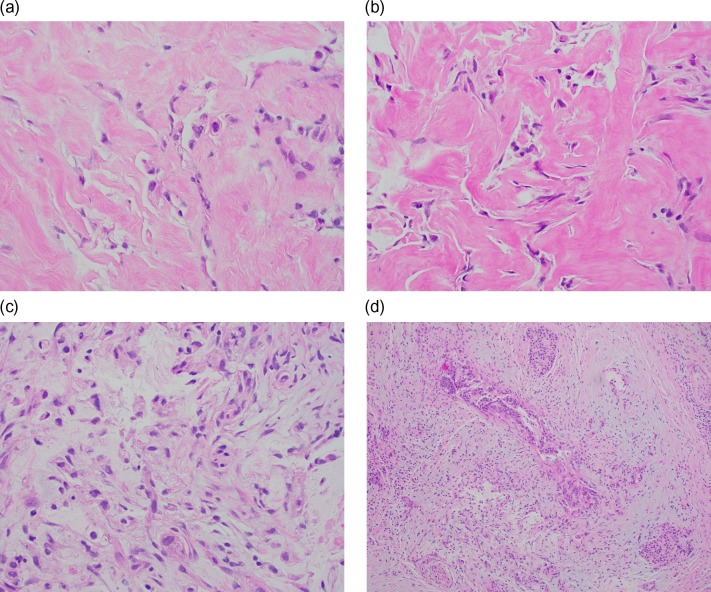
Figure 1:
(a) Signet ring cells present singly and in loose clusters within a desmoplastic and fibrotic stroma (H&E, ×400). (b) Signet ring cells seen in an extensive background of fibrosis (H&E, ×400). (c) Signet ring cells with vacuolated to clear cytoplasm noted in a desmoplastic stroma with some associated background mucin (H&E, ×400). (d) Chronic pancreatitis-like areas seen adjacent and admixed with the tumor cells (H&E, ×100).
DISCUSSION
The functional relationship between inflammation and cancer is well known, and an inflammatory component is present in the microenvironment of most neoplastic tissues [5]. Pancreatic ductal adenocarcinoma (PDAC) represents the quintessential example of an inflammation-driven cancer [3], however, most PDAC cases develop in the absence of clinically evident overt pancreatitis. The location of the organ in the retroperitoneal space masks early symptoms, and pancreatic cancer is frequently undetected until prominent clinical signs seem to abruptly appear. Further, the inaccessible portion of pancreas and the difficulty in distinguishing inflammatory changes from malignancy makes it difficult to establish a pre-operative diagnosis. Additionally, the insidious development of pancreatic carcinoma in the background of chronic pancreatitis poses a difficult diagnostic challenge because of overlap in clinical findings and often confusing imaging features.
Pathologically, the signet-ring cells are seen floating in abundant extracellular mucin pools either as single cells or in loose clusters [6], losing contact with the surrounding structures. In the gastrointestinal tract SRCC diffusely infiltrate throughout the bowel wall and often incite a marked desmoplastic reaction in the submucosa and muscularis propria [6]. They exhibit aggressive behavior with regard to invasion and metastasis. SRCC has two growth patterns. The focal pattern is predominately characterized by cystic spaces lined with malignant columnar epithelium filled with mucin, whereas the diffuse pattern consists of mucin lakes with clumps of less differentiated cells (Fig. 1) [7]. The histologic findings in our case were consistent with those described in previous case reports, where inflammatory cells were seen in pre-op biopsy subsequent definite diagnosis was established only during autopsy [7]. In cases diagnosed by EUS, we believe that signet ring cells obstructing the CBD, eroded the ducts to exfoliate into pancreatic secretions reaching the point of sampling [8, 9]. In cases of repeated negative EUS-FNA findings, a FNB specimen containing core tissue may provide a greater diagnostic accuracy as reported by Radojkovic et al. [10].
In conclusion, we report a case of SRCC of the pancreas which the diagnosis of cancer was delayed because multiple biopsies revealed only inflammatory changes with no obvious evidence of malignancy. To overcome these conflicting situations, based on suspicious clinical and imaging findings an aggressive surgical approach should be advocated to eradicate incipient lesions which can offer a better prognosis.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Lee NK, Kim S, Kim HS, Jeon TY, Kim GH, Kim DU, et al. Spectrum of mucin-producing neoplastic conditions of the abdomen and pelvis: cross-sectional imaging evaluation. World J Gastroenterol 2011;17:4757–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Acharya MN, Panagiotopoulos N, Cohen P, Ahmad R, Jiao LR. Poorly-differentiated signet-ring cell carcinoma of the ampulla of Vater: report of a rare malignancy. JOP 2013;14:190–4. [DOI] [PubMed] [Google Scholar]
- 3. Zambirinis CP, Pushalkar S, Saxena D, Miller G. Pancreatic cancer, inflammation, and microbiome. Cancer J 2014;20:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levy MJ. Endoscopic ultrasound-guided trucut biopsy of the pancreas: prospects and problems. Pancreatology 2007;7:163–6. [DOI] [PubMed] [Google Scholar]
- 5. Landskron G, De La Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014;2014:149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sung CO, Seo JW, Kim KM, Do IG, Kim SW, Park CK. Clinical significance of signet-ring cells in colorectal mucinous adenocarcinoma. Mod Pathol 2008;21:1533–41. [DOI] [PubMed] [Google Scholar]
- 7. Mcarthur CP, Fiorella R, Saran BM. Rare primary signet ring carcinoma of the pancreas. Mo Med 1995;92:298–302. [PubMed] [Google Scholar]
- 8. Karaahmet F, Basar O, Coban S, Aydog G, Yuksel O. Signet ring cell carcinoma of both colon and pancreas. J Gastrointest Cancer 2015;46:445–6. [DOI] [PubMed] [Google Scholar]
- 9. Terada T. Primary signet-ring cell carcinoma of the pancreas diagnosed by endoscopic retrograde pancreatic duct biopsy: a case report with an immunohistochemical study. Endoscopy 2012;44:E141–2. [DOI] [PubMed] [Google Scholar]
- 10. Radojkovic M, Ilic D, Ilic I. Primary signet ring cell carcinoma of the pancreas with a good response to chemotherapy: case report and literature review. Tumori 2017;103:e50–2. [DOI] [PubMed] [Google Scholar]