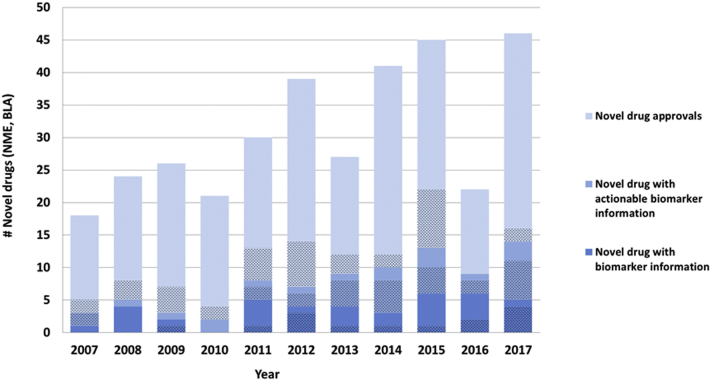
Figure 1.
Novel Drugs Approved (NME/BLA) Between 2007 and 2017 With Genomic and Other Selected Biomarker Information in Labeling
Shaded areas depict oncology drugs. Actionable biomarker information refers to a specific prescribing recommendation that is included in one of the following label sections: 1) Boxed Warning, 2) Indications and Usage, 3) Dosage and Administration, 4) Contraindications, or 5) Warnings and Precautions. Biomarkers may be any genomic biomarker or other selected protein biomarker that are used for patient selection. BLA = Biologic License Application; NME = New Molecular Entity.