Table 1. Optimization of reaction conditions a .
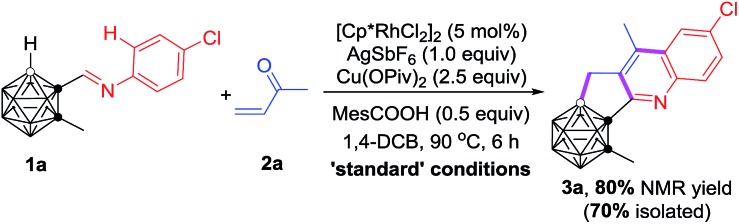
| ||
| Entry | Variations from the ‘standard’ conditions | Yield of 3a (%) |
| 1 | Without Cu(OPiv)2 | Trace |
| 2 | Cu(OAc)2 instead of Cu(OPiv)2 | 17 |
| 3 | Without MesCOOH | 68 |
| 4 | PivOH instead of MesCOOH | 74 |
| 5 | AgSbF6 (0.5 equiv.) | 53 |
| 6 | AgNTf2 instead of AgSbF6 | 9 |
| 7 | 80 °C instead of 90 °C | 34 |
| 8 | 100 °C instead of 90 °C | 74 |
| 9 | DCE instead of 1,4-DCB | Trace |
| 10 | Toluene instead of 1,4-DCB | — |
| 11 | [Ir] instead of [Rh] | Trace |
| 12 | [Ru] instead of [Rh] | 36 |
| 13 | [Rh] (2.5 mol%) | 70 |
| 14 | 2-Butenone (1.0 equiv.) | 33 |
| 15 | 2-Butenone (3.0 equiv.) | 62 |
| 16 | Cu(OPiv)2 (1.0 equiv.) | 49 |
| 17 | Cu(OPiv)2 (2.0 equiv.) | 71 |
| 18 | Under air | 39 |
aReaction conditions: 1a (0.05 mmol) and 2a (0.25 mmol) in 1.5 mL of solvent under argon in a closed flask; 1,4-DCB = 1,4-dichlorobutane; Cu(OPiv)2 = copper pivalate; AgSbF6 = silver hexafluoroantimonate(V); MesCOOH = 2,4,6-trimethylbenzoic acid; PivOH = pivalic acid; AgNTf2 = silver bis(trifluoromethanesulfonyl)imide; DCE = 1,2-dichloroethane; [Ir] = [Cp*IrCl2]2; [Ru] = [Ru(p-cymene)Cl2]2. Yield determined by 1H NMR spectroscopy using dibromomethane as an internal standard.
