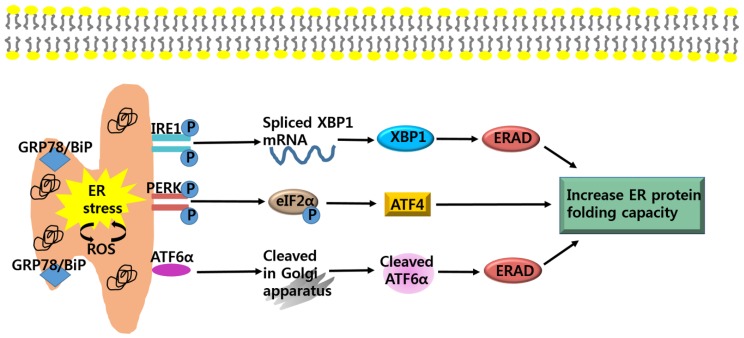
Figure 1.
Adaptive pathways of endoplasmic reticulum (ER) stress. ER stress is induced by an accumulation of unfolded proteins in the ER lumen. During ER stress, glucose-regulated protein 78 (GRP78)/ binding protein (BiP) dissociates from its interaction with the three ER stress sensors, inositol-requiring protein 1 (IRE1), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6), which become activated. IRE1 mediates splicing of α-x-box binding protein 1 (XBP1), which is responsible for the upregulation of ER associated degradation (ERAD). PERK phosphorylates eIF2α and induce activating transcription factor 4 (ATF4), which is involved in restoring ER homeostasis. ATF6α cleaved by specific Golgi resident proteases, increase expression of UPR genes and ERAD. Eukaryotic initiation factor 2 (eIf2); protein kinase RNA-like ER kinase (PERK).