ABSTRACT
Magnaporthe oryzae is a filamentous fungus, which causes significant destruction to cereal crops worldwide. To infect plant cells, the fungus develops specialised constricted structures such as the penetration peg and the invasive hyphal peg. Live-cell imaging of M. oryzae during plant infection reveals that nuclear migration occurs during intermediate mitosis, in which the nuclear envelope neither completely disassembles nor remains entirely intact. Remarkably, in M. oryzae, mitotic nuclei show incredible malleability while undergoing confined migration through the constricted penetration and invasive hyphal pegs. Here, we review early events in plant infection, discuss intermediate mitosis, and summarise current knowledge of intermediate mitotic nuclear migration in M. oryzae.
KEYWORDS: Appressoria, intermediate mitosis, Magnaporthe oryzae, mitosis, nuclear envelope, nuclear migration, nuclear plasticity, rice blast, semi-closed mitosis
Introduction
Magnaporthe oryzae, also known as the rice blast fungus, is a filamentous hemibiotrophic plant pathogen. It is capable of mass destruction to valuable plant crops such as rice and wheat, as well as barley, finger millet, and foxtail millet (Gladieux et al. 2018). In fact, each year M. oryzae causes an estimated $66 billion in economic damage to rice crops, destroying enough food to have fed 60 million people (Pennisi 2010). In the field, M. oryzae is developing increased resistance to commonly used fungicides (Ribas e Ribas et al. 2016) and, recently, wheat blast emerged in Bangladesh (Islam et al. 2016; Malaker et al. 2016). Understanding cellular processes unique to M. oryzae is an important first step in the development of novel and effective methods to control the deadly plant pathogen and ensure global food security.
Proper positioning of the nucleus within eukaryotic cells is vital and relies upon successful nuclear migration into incipient cells (Morris 2000). A full gamut of mitotic forms is possible in eukaryotes, ranging from completely closed to completely open (Heath 1980; Boettcher and Barral 2013). In fungi, nuclear migration into incipient cells occurs before, during, or after mitosis (Gladfelter and Berman 2009). Recent studies reveal that M. oryzae undergoes mitosis that is not completely closed or open and that the mitotic nucleus becomes highly deformed while migrating through narrow structures that arise during plant infection (Jones et al. 2016a; Jenkinson et al. 2017).
In this review, we highlight the nuclear dynamics of M. oryzae during plant infection with a focus on mitosis and mitotic nuclear migration. We contextualise these cellular processes by discussing the early events in rice blast infection and the range of mitotic programmes documented in fungi. We provide an outlook on what mechanisms of nuclear migration likely exist in M. oryzae and discuss whether similar cellular processes are present in other plant pathogens.
Early events in rice blast infection
Rice blast infection begins when an asexual three-celled conidium attaches to the surface of a rice plant (Hamer et al. 1988). A polarised germ tube develops, and the fungus forms a melanised dome-shaped cell called an appressorium in the presence of the appropriate extracellular physical and chemical signals, for instance, hydrophobicity of the leaf surface (Veneault-Fourrey et al. 2006; Ryder and Talbot 2015). The most apical nucleus of the conidium undergoes mitosis, and one nucleus migrates to the incipient appressorium, followed by autophagy of the conidium (Veneault-Fourrey et al. 2006; Saunders et al. 2010a). It remains unknown if extracellular cues trigger mitosis during appressorium development. Accumulation of turgor pressure in the appressorium in coordination with septin-dependent cytoskeletal rearrangements at the appressorial pore leads to formation of the penetration peg (Howard et al. 1991; Dagdas et al. 2012). The penetration peg is a specialised hypha that physically breaches the leaf cuticle, allowing the fungus to enter rice cells approximately 24 h post-inoculation (Kankanala et al. 2007). Several S-phase checkpoints have been identified, which regulate appressorium development and formation of the penetration peg (Saunders et al. 2010a; Osés-Ruiz et al. 2017). Once inside the first-invaded rice cell, the penetration peg gives rise to the primary hypha (Kankanala et al. 2007). The apical tip of the primary hypha switches from filamentous to depolarised growth causing the apical tip to swell (Shipman et al. 2017). Tip expansion of the primary hypha appears to serve as a size threshold which triggers mitosis in the single nucleus located in the appressorium (Shipman et al. 2017). This nucleus begins mitosis inside the appressorium and undergoes a long-distance migration during presumed anaphase B to its eventual position in the swollen tip of the primary hypha (Jenkinson et al. 2017; Shipman et al. 2017). Subsequently, septation occurs, and the first cell of the bulbous invasive hyphae (IH) is formed (Shipman et al. 2017). Incongruent descriptions of the exact location of mitosis at this infection stage exist in the literature. Two previous reports describe the appressorial nucleus migrating from the appressorium into the primary hypha and then undergoing mitosis, rendering the appressorium anucleate for a time (Veneault-Fourrey et al. 2006; Fernandez et al. 2014). However, later work reports mitosis as most commonly occurring within the appressorium (Jenkinson et al. 2017; Osés-Ruiz et al. 2017; Shipman et al. 2017). The reason for this discrepancy remains unknown.
M. oryzae secretes effector proteins during plant infection. Effectors function to dampen plant immune responses and change the metabolism of the plant (Giraldo et al. 2013). The biotrophic interfacial complex (BIC) is a plant-derived membrane-rich structure that lies outside the fungal cytoplasm and is the site at which cytoplasmic effectors accumulate during plant infection (Khang et al. 2010; Giraldo et al. 2013). The BIC first appears at the tip of the primary hypha but then is repositioned to the side of the first bulbous cell (Khang et al. 2010; Shipman et al. 2017). Cytoplasmic effectors are secreted to the BIC via non-conventional secretion and eventually enter plant cells, while apoplastic effectors undergo conventional secretion and remain contained within the extra-invasive hyphal membrane (Giraldo et al. 2013).
To invade neighbouring plant cells, IH appear to seek out pit fields and undergo a morphological shift from polarised to isotropic growth controlled by the Pmk1 mitogen-activated protein kinase (Kankanala et al. 2007; Sakulkoo et al. 2018). IH then form highly constricted IH pegs to cross plant cell walls (Kankanala et al. 2007). The first-invaded rice cells are alive but die when the fungus enters adjacent cells (Kankanala et al. 2007; Jones et al. 2016b) Colonisation of the first-invaded plant cell takes approximately 8–12 h, while in subsequently invaded cells, the fungus only develops for 2–3 h (Kankanala et al. 2007; Jones et al. 2016b). One possible explanation for the difference in colonisation time between first-invaded and subsequently invaded cells is that effectors move cell-to-cell likely through plasmodesmata and prime neighbouring cells for fungal invasion (Kankanala et al. 2007; Khang et al. 2010). Within 4–5 days of initial infection, blast lesions become apparent on the plant tissue (Sakulkoo et al. 2018).
The mitotic spectrum in fungi
A fundamental property of eukaryotic life is the division of replicated genetic information to new daughter cells through mitosis. Typically, when we think of mitosis, we recall cellular events characteristic of mitosis in plant and animal cells that use completely open mitosis where the nuclear envelope (NE) disassembles during prophase. However, multiple forms of mitosis exist across the eukaryotic domain (Arnone et al. 2013; Sazer et al. 2014; Makarova and Oliferenko 2016). In fungi, it is often assumed that all species rely on completely closed mitosis, yet, closer study of mitotic nuclear dynamics reveal a spectrum of mitotic programmes in fungi (Heath 1980; De Souza and Osmani 2007).
Mitosis is categorised based on the state of the NE during nuclear division (Arnone et al. 2013; Sazer et al. 2014; Makarova and Oliferenko 2016). In completely closed mitosis, the NE remains entirely intact throughout nuclear division. Spindle pole bodies, embedded within the NE, serve as microtubule-organising centres and permit the spindle to form within the nucleus (Arnone et al. 2013). The spindle elongates in coordination with NE expansion, and eventually the condensed chromosomes are separated. Conversely, in completely open mitosis, all components of the NE undergo systematic dismantling during prophase (Arnone et al. 2013). This regulated disassembly of the NE grants the spindle, which is nucleated in the cytoplasm, access to chromosomes which are then separated. The NE is reformed at the end of mitosis, enclosing two daughter nuclei.
Historically, classification of closed or open mitosis was determined by observing the state of the NE using transmission electron microscopy throughout all phases of mitosis in classical model organisms, such as the closed mitosis of Saccharomyces cerevisiae (Sazer et al. 2014). As studies of NE dynamics during mitosis expanded beyond model organisms, it became obvious that classifying mitosis as either completely closed or completely open is not always clear, especially in fungi (Heath 1980). The NE of some species persists throughout mitosis but is not fully intact, thus exhibiting characteristics of both a closed and open mitosis (Heath 1980; Sazer et al. 2014). Mitosis that is not completely closed nor completely open is called intermediate mitosis (De Souza and Osmani 2007; Arnone et al. 2013). Intermediate mitosis involves changes to the structure and integrity of the NE, which leads to a significant loss of compartmentalisation between the nucleus and the cytoplasm. Several terms exist to describe forms of intermediate mitosis, for instance, semi-open in Aspergillus nidulans (Lin et al. 2015), modified-open in Ustilago maydis (Straube et al. 2005), and partially open as a collective term for all forms of intermediate mitosis (Arnone et al. 2013; Sazer et al. 2014). Generally, mechanisms of intermediate mitosis in fungi can be grouped into two broad categories: tearing of the NE or altering the composition of a largely intact NE to enhance permeability. Fungi such as U. maydis and Schizosaccharomyces japonicus experience NE tearing during mitosis (Straube et al. 2005; Aoki et al. 2011; Yam et al. 2011). In A. nidulans, a subset of proteins found within nuclear pore complexes (NPCs) disperse into the cytoplasm during mitosis (De Souza et al. 2004; Osmani et al. 2006).
Intermediate mitosis in Magnaporthe oryzae
Mitosis in M. oryzae bears a hallmark of intermediate mitosis, the dramatic loss of compartmentalisation between the nucleus and the cytoplasm. During mitosis, cytoplasmic ZsGreen signal equalises between the cytoplasm and the nucleus (Bourett et al. 2002), and the import of tubulin into the nucleus increases dramatically after mitotic onset (Czymmek et al. 2005). Following the dynamics of green fluorescent protein fused with nuclear localisation signal (GFP-NLS) during plant infection provides further evidence of intermediate mitosis (Jones et al. 2016a; Jenkinson et al. 2017). GFP-NLS signal remains within the nucleus during interphase in the appressorium and IH. However, at the start of mitosis, GFP-NLS signal becomes cytoplasmic with subsequent reimportation of GFP-NLS once mitosis is complete (Figure 1). Together, these reports show that mitosis in M. oryzae is not completely closed where the NE would be expected to function as an intact barrier to prevent mixing of nuclear and cytoplasmic proteins. Possible explanations for this observed loss of compartmentalisation include (1) the NE of M. oryzae tears or (2) the NE persists but loses integrity during mitosis. Differentiating between these two possibilities requires following the dynamics of the NE throughout mitosis.
Figure 1.

Time-lapse confocal fluorescence images and schematic diagram of intermediate mitosis in an invasive hypha of M. oryzae within the first-invaded rice cell. This strain expresses histone H1-tdTomato and GFP-NLS. The top panel shows five sequential fluorescence pattern stages in both merged bright-field and fluorescence (top), red fluorescence alone (middle), and green fluorescence alone (bottom). The interphase nucleus in G2 appears yellow due to colocalization of H1-tdTomato (red) and GFP-NLS (green) within the nucleus. H1-tdTomato remains associated with DNA throughout the cell cycle. During the early stages of mitosis (M), GFP-NLS spills into the cytoplasm, indicating a change to the integrity of the nuclear envelope. GFP-NLS is contained within the dividing cell by presumed closure of septal pores. Following mitosis, GFP-NLS is reimported back into the nucleus and the nucleus again becomes yellow during interphase (G1). The bottom panel presents a schematic summary of these cellular events. Bar = 5 μm. This figure is modified from Jones et al. (2016a). To view this figure in color, please see the online version of this journal.
Staining the outer nuclear membrane using the lipophilic dye 3,3ʹ-dihexyloxacarbocyanine iodide showed that the outer nuclear membrane remains intact during mitosis in the germ tube at the appressorium development stage of infection (Saunders et al. 2010b). Additional studies of the NE at the same infection stage reveal that the core nucleoporin (Nup), Nup84, localises to the polar edges of the dividing nucleus (Pfeifer and Khang, unpublished data). The persistence of Nup84 at the NE throughout mitosis confirms that portions of the NE do remain intact during nuclear division. Additionally, the polar localisation of Nup84 in M. oryzae is similar to other fungi known to use intermediate mitosis, including S. japonicus (Aoki et al. 2011; Yam et al. 2011) and A. nidulans (Osmani et al. 2006). While these results confirm distinct components of the NE remain intact during mitosis, the details of the mechanism responsible for intermediate mitosis remain to be discovered in M. oryzae.
Interestingly, M. oryzae shares many mitotic similarities with A. nidulans, a model organism with well-studied mitotic dynamics. The NE of both species remains intact throughout mitosis (Robinow and Caten 1969; Saunders et al. 2010b) while the permeability of the NE increases during mitosis (Suelmann et al. 1997; Bourett et al. 2002; Ovechkina et al. 2003; De Souza et al. 2004; Czymmek et al. 2005; Osmani et al. 2006; Jones et al. 2016a; Shipman et al. 2017). Furthermore, A. nidulans and M. oryzae show loss of compartmentalisation between the nucleus and cytoplasm with a coordinated closure of septal pores during mitosis (Ovechkina et al. 2003; De Souza et al. 2004; Osmani et al. 2006; Shen et al. 2014; Jones et al. 2016a). In M. oryzae, cytoplasmic GFP-NLS remains contained within the dividing cell during mitosis, which suggests cell cycle regulation of septal pores (Jones et al. 2016a). A similar case is observed in A. nidulans where the NIMA kinase coordinates septal pore opening and closing to be out of sync with the dispersal of Nups from NPCs during prophase (Shen et al. 2014). This regulated closure of septal pores likely prevents diffusion of mitotic kinases throughout neighbouring cells thereby preventing precocious mitoses which could be detrimental to the fungus (Shen et al. 2014). Future study of the NIMA homolog will reveal if similar regulation of septal pores exists in M. oryzae.
Although mounting evidence strongly suggests that M. oryzae uses a form of intermediate mitosis, details about the process are lacking. Data describing the dynamics of all components of the NE throughout mitosis is needed to fully characterise the form of intermediate mitosis used by M. oryzae. To date, only the localisation of the outer nuclear membrane and Nup84 has been studied during mitosis at one stage of rice blast infection, appressorium development. An important direction for future research is to visualise the inner nuclear membrane using a combination of confocal and transmission electron microscopy throughout mitosis to fully describe whether this component of the NE remains intact during mitosis in M. oryzae. Given the mitotic similarities to A. nidulans, we hypothesise that a key feature of mitosis in M. oryzae is dispersal of peripheral Nups during prophase while other NE components (the outer and inner nuclear membranes along with core Nups) remain intact during mitosis. Demonstrating that core Nups remain associated with the NE during mitosis while peripheral Nups localise to the cytoplasm will implicate a mechanism akin to A. nidulans (Osmani et al. 2006).
Although we hypothesise M. oryzae shares key mitotic features with A. nidulans, it is important to note that nuclear distribution differs between the two species. M. oryzae is typically a mononuclear species, with only one nucleus per cell. A. nidulans, however, has multiple nuclei within one common cytoplasm. Since A. nidulans is syncytial, the persistence of the NE may help prevent spindle microtubules from interacting with the chromosomes of a nearby dividing nucleus in an adulterous manner (De Souza and Osmani 2007). Why then does M. oryzae use intermediate mitosis? The variety of mitotic programmes present in fungi suggests that each type of mitosis conveys advantages and disadvantages to the organism (Boettcher and Barral 2013). We speculate that intermediate mitosis in M. oryzae confers a to-be-determined advantage, perhaps to permit more efficient plant infection. However, research in this area is very much in its nascence. Future work in M. oryzae may better address what advantages intermediate mitosis brings to fungi during plant infection.
Nuclear constrictions during plant infection
M. oryzae’s IH are highly plastic as they grow inside plant cells. For instance, during cell-to-cell movement as IH cross from the first-invaded cell to adjacent cells via the narrow IH peg, IH constrict from an initial average diameter of 5 to 0.5 μm (Kankanala et al. 2007; Sakulkoo et al. 2018). Live-cell imaging of cell-to-cell movement revealed that hyphae developing in the newly invaded neighbour cell must grow significantly before becoming nucleated (Kankanala et al. 2007). These results suggested that nucleation of the incipient fungal hypha depends upon mitosis and the successful delivery of the daughter nucleus across the IH peg. Until recently, the details of this process remained enigmatic, largely due to the technical challenges of capturing rapid nuclear migration through the IH peg using time-lapse imaging.
An elegant combination of GFP-NLS and histone H1-tdTomato fluorescent reporter proteins was utilised to study nuclear dynamics during plant infection. GFP-NLS spill from the nucleus into the cytoplasm indicates entrance into prophase and that the nucleus will divide within a few minutes, while histone H1-tdTomato remains associated with DNA throughout the cell cycle (Jones et al. 2016a; Jenkinson et al. 2017). This combination of fluorescent proteins provided crucial temporal and spatial cues to time-lapse image nuclear migration events inside the rice plant without causing phototoxicity to divide fungal cells (Jones et al. 2016a; Jenkinson et al. 2017). Using GFP-NLS as an indicator for mitosis demonstrates that nuclear migration occurs during intermediate mitosis. That is, GFP-NLS disperses from the nucleus before migration occurs and is not fully reimported back until after nuclear migration (Jones et al. 2016a; Jenkinson et al. 2017).
These studies further revealed several important findings about nuclear migration during plant infection (Figure 2 and full videos are available on youtube.com by searching Khang Lab at UGA Rice Blast). Firstly, the nucleus adopts an extremely constricted morphology as it migrates through the IH peg. One nucleus (interphase diameter of ~2 μm) was observed to stretch to over 5 μm in length while moving through the IH peg (Jones et al. 2016a). After squeezing through the IH peg, the nucleus assumed its typical spherical shape and continued to migrate a total distance of 16.9 μm from the site of chromosome separation to the now nucleated fungal hyphal cell located in the second-invaded rice cell (Jones et al. 2016a). This remarkable nuclear morphology was also observed at an earlier stage of plant infection, when the appressorial nucleus moved through the penetration peg (diameter of .7 μm) during mitosis (Jenkinson et al. 2017; Shipman et al. 2017). Here, the appressorial nucleus became highly constricted and elongated with a maximum length of 13 μm reported (Jenkinson et al. 2017). At both stages of infection, the migrating nucleus appeared to be tethered to the mother nucleus (Jones et al. 2016a; Jenkinson et al. 2017). Notably, only the migrating nucleus could enter and cross the penetration or IH peg. Together, these observations suggest that M. oryzae possesses a cellular mechanism responsible for the confined migration of the mitotic nucleus through narrow pegs arising during plant infection.
Figure 2.
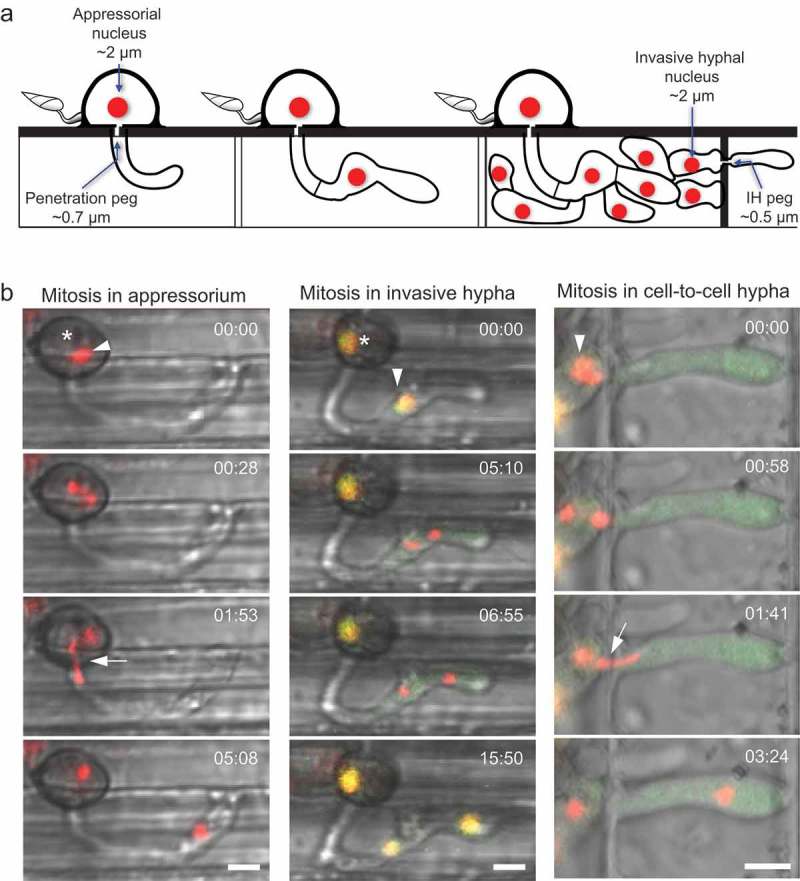
Mitotic migration of M. oryzae nuclei during early rice blast infection. (a) Schematic diagram summarizing key cellular structures and mononuclear positioning during plant invasion. The nucleus in the appressorium (interphase diameter of ~2 μm) must traverse the constricted penetration peg (diameter of ~0.7 μm) for final receipt in the incipient primary hypha. Once inside the first-invaded cell, the primary hypha becomes bulbous to form invasive hyphae (IH). To move into adjacent rice cells, IH seek out pit fields and develop a constricted IH peg (diameter of ~0.5 μm). (b) A time-lapse series of nuclear dynamics at three distinct stages of early rice blast infection. Asterisks denote the appressorium, arrowheads label a nucleus about to undergo mitotic nuclear migration, and arrows highlight extreme nuclear morphology during confined nuclear migration through peg structures. (Left: merge of bright-field and H1-tdTomato.) Mitosis begins in the appressorium, and the daughter nucleus becomes highly constricted and elongated during confined mitotic nuclear migration through the penetration peg. The original nucleus remains located in the appressorium throughout this event. GFP-NLS dynamics (data not shown) confirms nuclear migration occurs during intermediate mitosis at this infection stage (Jenkinson et al. 2017). (Middle panel: merge of bright-field, GFP-NLS, and H1-tdTomato.) During mitosis in the invasive hypha, the interphase nucleus appears yellow due to colocalisation of H1-tdTomato and GFP-NLS in the nucleus. After onset of mitosis, GFP-NLS disperses into the cytoplasm, and the nucleus undergoes an unconfined nuclear migration. Following receipt of the nucleus into the new invasive hypha cell, mitosis ends, and GFP-NLS is fully reimported back into the nucleus. (Right panel: merge of bright-field, GFP-NLS, and H1-tdTomato.) Here, confined nuclear migration through the IH peg occurs. In early mitosis, the sister chromatids separate and during presumed anaphase B, a single daughter nucleus undergoes confined nuclear migration through the constricted IH peg. The daughter nucleus again becomes spherical and continues to migrate to the tip of the IH in the second-invaded cell prior to GFP-NLS reimport into the nucleus. Times are shown in minutes:seconds. Bars = 5 μm. This figure is modified from Jones et al. (2016a) and Jenkinson et al. (2017).
The ability of M. oryzae’s nuclei to withstand such extreme constrictions during plant infection is certainly captivating. Fungal nuclei are known to be flexible, largely attributed to the fact that fungi lack true lamin proteins (Steinberg et al. 2012; Ciska and Moreno Díaz De La 2014). The nuclei of fungi such as U. maydis (Straube et al. 2005), Neurospora crassa (Roca et al. 2010), and Candida albicans (Finley and Berman 2005) all display some degree of elongation during migration. However, to our knowledge, the morphology adopted by the nuclei of M. oryzae during confined migration through the penetration or IH peg is the most drastic morphology to be reported.
Nuclear migration during plant infection
Nuclear migration in many fungi requires the coordination of microtubules, the motor protein cytoplasmic dynein, and additional microtubule-associated proteins; for extensive reviews of this topic, see Gladfelter and Berman (2009), Roberts and Gladfelter (2016), and Xiang (2018). For example, in S. cerevisiae, astral microtubules are nucleated at the spindle pole body and rely on dynamic instability to search the cell cortex of the bud to locate Num1, a cortical dynein-interacting protein (Carminati and Stearns 1997; Heil-Chapdelaine et al. 2000; Farkasovsky and Küntzel 2001). Once the plus-ends of astral microtubules bind to Num1, they slide against the cell cortex in a dynein-mediated manner which moves the spindle and eventually the daughter nucleus into the newly formed bud (Heil-Chapdelaine et al. 2000). Functional homologs of Num1 are also required for proper nuclear migration in multinucleate fungi, including A. nidulans (Veith et al. 2005) and Ashbya gossypii (Grava et al. 2011). In M. oryzae, a Num1 homolog, MoAND1, has been characterised (Jeon et al. 2014). Without MoAND1, nuclear positioning in vegetative hyphae and conidia is affected (Jeon et al. 2014). Importantly, ΔMoand1 shows a reduced ability to initially invade rice cells, suggesting that a cortical dynein anchor is necessary for the fungus to penetrate plants.
A recent study of a class myosin-II motor, Momyo2, in M. oryzae showed that disruption of Momyo2 resulted in aberration in nuclear distribution (Guo et al. 2017). Like the ΔMoAND1, the ΔMomyo2 strain shows reduced ability to penetrate into plants (Guo et al. 2017). In other fungi, class-II myosins in cooperation with actin play important roles in cytokinesis and septation (Takeshita 2016). Future research investigating the functions of microtubules and actin along with associated motor proteins will yield valuable information about M. oryzae’s mechanism of nuclear migration during plant infection.
Outlook and conclusion
We now know that M. oryzae nuclei become extremely constricted while migrating through the confined channels of the penetration or IH peg and that nuclear migration occurs during intermediate mitosis. Does the NE regularly rupture during mitotic migration? Is NE rupture more frequent as the nucleus migrates through the pegs? What are the motor and accessory proteins needed for successful nuclear migration during plant infection? The answers to these intriguing questions remain to be discovered.
Interestingly, other fungi also form confined structures during plant infection. For example, Colletotrichum spp. form an appressorium-derived penetration peg to initially enter their host cells (Mendgen et al. 1996; Nesher et al. 2008; De Silva et al. 2017). Fusarium graminearum forms intracellular hyphae that exhibit apparent constriction during cell-to-cell movement (Jansen et al. 2005). Although it is currently unknown whether F. graminearum uses intermediate mitotic nuclear migrations during infection, some other Fusarium spp., such as F. oxysporum and F. verticillioides, show signs of intermediate mitosis (Bourett et al. 2002; De Souza and Osmani 2007). Future work in filamentous fungi including M. oryzae will provide evidence needed to draw broader conclusions regarding conserved mechanisms of mitotic nuclear migration during plant infection.
As technology advances to allow studies of cellular processes at single-cell resolution, an important aim will be to discover how intracellular fungal pathogens position nuclei during plant infection. M. oryzae represents one of the most significant threats to global food production, and resistance to fungicides such as azoles is increasing (Ribas e Ribas et al. 2016). Investigating nuclear migration in M. oryzae during plant infection will likely identify fungal-specific cellular targets to halt nuclear migration and thereby prevent infection progression. Insight into these fascinating cellular mechanisms could aid in the development of new fungicides to control the deadly rice blast fungus and other fungal plant pathogens.
Funding Statement
This work was supported by the Agriculture and Food Research Initiative competitive grants program, award number 2014-67013-21717, from the USDA National Institute of Food and Agriculture.
Acknowledgements
We thank all members of the Khang Lab (http://www.khanglab.org/) for their help and discussion. A special thank-you to current member Kiersun Jones for generating Figure 2. This work was supported by the Agriculture and Food Research Initiative competitive grants program, award number 2014-67013-21717, from the USDA National Institute of Food and Agriculture.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aoki K, Hayashi H, Furuya K, Sato M, Takagi T, Osumi M, Kimura A, Niki H.. 2011. Breakage of the nuclear envelope by an extending mitotic nucleus occurs during anaphase in Schizosaccharomyces japonicus. Genes to Cells. 16:911–926. [DOI] [PubMed] [Google Scholar]
- Arnone JT, Walters AD, Cohen-Fix O.. 2013. The dynamic nature of the nuclear envelope. Nucleus. 4:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher B, Barral Y. 2013. The cell biology of open and closed mitosis. Nucleus. 4:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourett TM, Sweigard JA, Czymmek KJ, Carroll A, Howard RJ. 2002. Reef coral fluorescent proteins for visualizing fungal pathogens. Fungal Genet Biol. 37:211–220. [DOI] [PubMed] [Google Scholar]
- Carminati JL, Stearns T. 1997. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J Cell Biol. 138:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciska M, Moreno Díaz de La ES. 2014. The intriguing plant nuclear lamina. Front Plant Sci. 5:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza CPC, Osmani SA. 2007. Mitosis, not just open or closed. Eukaryot Cell. 6:1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czymmek KJ, Bourett TM, Shao Y, DeZwaan TM, Sweigard JA, Howard RJ. 2005. Live-cell imaging of tubulin in the filamentous fungus Magnaporthe grisea treated with anti-microtubule and anti-microfilament agents. Protoplasma. 225:23–32. [DOI] [PubMed] [Google Scholar]
- Dagdas YF, Yoshino K, Dagdas G, Ryder LS, Bielska E, Steinberg G, Talbot NJ. 2012. Septin-mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science. 336:1590–1595. [DOI] [PubMed] [Google Scholar]
- De Silva DD, Crous PW, Ades PK, Hyde KD, Taylor PWJ. 2017. Life styles of colletotrichum species and implications for plant biosecurity. Fungal Biol Rev. 31:155–168. [Google Scholar]
- De Souza CPC, Osmani AH, Hashmi SB, Osmani SA. 2004. Partial nuclear pore complex disassembly during closed mitosis in Aspergillus nidulans. Curr Biol. 14:1973–1984. [DOI] [PubMed] [Google Scholar]
- Farkasovsky M, Küntzel H. 2001. Cortical num1p interacts with the dynein intermediate chain pac11p and cytoplasmic microtubules in budding yeast. J Cell Biol. 152:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J, Marroquin-Guzman M, Wilson RA. 2014. Evidence for a transketolase-mediated metabolic checkpoint governing biotrophic growth in rice cells by the blast fungus Magnaporthe oryzae. PLoS Pathog. 10:e1004354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley KR, Berman J. 2005. Microtubules in candida albicans hyphae drive nuclear dynamics and connect cell cycle progression to morphogenesis. Eukaryot Cell. 4:1697–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo MC, Dagdas YF, Gupta YK, Mentlak TA, Yi M, Martinez-Rocha AL, Saitoh H, Terauchi R, Talbot NJ, Valent B. 2013. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat Commun. 4:1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter A, Berman J. 2009. Dancing genomes: fungal nuclear positioning. Nat Rev Microbiol. 7:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladieux P, Condon B, Ravel S, Soanes D, Maciel JLN, Nhani A, Chen L, Terauchi R, Lebrun M-H, Tharreau D, et al. 2018. Gene flow between divergent cereal- and grass-specific lineages of the rice blast fungus Magnaporthe oryzae. MBio. 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grava S, Keller M, Voegeli S, Seger S, Lang C, Philippsen P. 2011. Clustering of nuclei in multinucleated hyphae is prevented by dynein-driven bidirectional nuclear movements and microtubule growth control in Ashbya gossypii. Eukaryot Cell. 10:902–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Tan L, Nie X, Zhang Z. 2017. A class-II myosin is required for growth, conidiation, cell wall integrity and pathogenicity of Magnaporthe oryzae. Virulence. 8:1335–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer JE, Howard RJ, Chumley FG, Valent B. 1988. A mechanism for surface attachment in spores of a plant pathogenic fungus. Science. 239:288–290. [DOI] [PubMed] [Google Scholar]
- Heath IB. 1980. Variant mitoses in lower eukaryotes: indicators of the evolution of mitosis? In: Bourne GH, Danielli JF, Jeon KW, editors. International review of cytology. Academic Press; p. 1–80. [DOI] [PubMed] [Google Scholar]
- Heil-Chapdelaine RA, Oberle JR, Cooper JA. 2000. The cortical protein num1p is essential for dynein-dependent interactions of microtubules with the cortex. J Cell Biol. 151:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RJ, Ferrari MA, Roach DH, Money NP. 1991. Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc Natl Acad Sci U S A. 88:11281–11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MT, Croll D, Gladieux P, Soanes DM, Persoons A, Bhattacharjee P, Hossain MS, Gupta DR, Rahman MM, Mahboob MG, et al. 2016. Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol. 14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen C, von Wettstein D, Schäfer W, Kogel K-H, Felk A, Maier FJ. 2005. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc Natl Acad Sci U S A. 102:16892–16897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson CB, Jones K, Zhu J, Dorhmi S, Khang CH. 2017. The appressorium of the rice blast fungus Magnaporthe oryzae remains mitotically active during post-penetration hyphal growth. Fungal Genet Biol. 98:35–38. [DOI] [PubMed] [Google Scholar]
- Jeon J, Rho H, Kim S, Kim KS, Lee Y-H. 2014. Role of MoAND1-mediated nuclear positioning in morphogenesis and pathogenicity in the rice blast fungus, Magnaporthe oryzae. Fungal Genet Biol. 69:43–51. [DOI] [PubMed] [Google Scholar]
- Jones K, Jenkinson CB, Borges Araújo M, Zhu J, Kim RY, Kim DW, Khang CH. 2016a. Mitotic stopwatch for the blast fungus Magnaporthe oryzae during invasion of rice cells. Fungal Genet Biol. 93:46–49. [DOI] [PubMed] [Google Scholar]
- Jones K, Kim DW, Park JS, Khang CH. 2016b. Live-cell fluorescence imaging to investigate the dynamics of plant cell death during infection by the rice blast fungus Magnaporthe oryzae. BMC Plant Biol. 16:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankanala P, Czymmek K, Valent B. 2007. Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell. 19:706–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khang CH, Berruyer R, Giraldo MC, Kankanala P, Park S-Y, Czymmek K, Kang S, Valent B. 2010. Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell. 22:1388–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Osmani AH, Osmani SA. 2015. The inner nuclear membrane protein src1 is required for stable post-mitotic progression into G1 in Aspergillus nidulans. PLoS One. 10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova M, Oliferenko S. 2016. Mixing and matching nuclear envelope remodeling and spindle assembly strategies in the evolution of mitosis. Curr Opin Cell Biol. 41:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaker PK, Barma NCD, Tiwari TP, Collis WJ, Duveiller E, Singh PK, Joshi AK, Singh RP, Braun HJ, Peterson GL, et al. 2016. First report of wheat blast caused by Magnaporthe oryzae pathotype triticum in Bangladesh. Plant Dis. 100:2330. [Google Scholar]
- Mendgen K, Hahn M, Deising H. 1996. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu Rev Phytopathol. 34:367–386. [DOI] [PubMed] [Google Scholar]
- Morris NR. 2000. Nuclear migration: from fungi to the mammalian brain. J Biol. 148:1097–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesher I, Barhoom S, Sharon A. 2008. Cell cycle and cell death are not necessary for appressorium formation and plant infection in the fungal plant pathogen Colletotrichum gloeosporioides. BMC Biol. 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osés-Ruiz M, Sakulkoo W, Littlejohn GR, Martin-Urdiroz M, Talbot NJ. 2017. Two independent S-phase checkpoints regulate appressorium-mediated plant infection by the rice blast fungus Magnaporthe oryzae. Proc Natl Acad Sci. 114:E237–E244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani AH, Davies J, Liu H-L, Nile A, Osmani SA, Solomon M. 2006. Systematic deletion and mitotic localization of the nuclear pore complex proteins of Aspergillus nidulans. Mol Biol Cell. 17:4946–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovechkina Y, Maddox P, Oakley CE, Xiang X, Osmani SA, Salmon ED, Oakley BR. 2003. Spindle formation in Aspergillus is coupled to tubulin movement into the nucleus. Mol Biol Cell. 14:2192–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi E. 2010. Armed and dangerous. Science. 327:804–805. [DOI] [PubMed] [Google Scholar]
- Ribas e Ribas AD, Spolti P, Del PEM, Donato KZ, Schrekker H, Fuentefria AM. 2016. Is the emergence of fungal resistance to medical triazoles related to their use in the agroecosystems? A mini review. Braz J Microbiol. 47:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SE, Gladfelter AS. 2016. Chapter 2, nuclear dynamics and cell growth in fungi In: Wendland J, editor. Growth, differentiation and sexuality. 3rd ed. Switzerland: Springer; p. 27–46. [Google Scholar]
- Robinow C, Caten C. 1969. Mitosis in Aspergillus nidulans. J Cell Sci. 5:403–431. [DOI] [PubMed] [Google Scholar]
- Roca MG, Kuo H-C, Lichius A, Freitag M, Read ND. 2010. Nuclear dynamics, mitosis, and the cytoskeleton during the early stages of colony initiation in Neurospora crassa. Eukaryot Cell. 9:1171–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder LS, Talbot NJ. 2015. Regulation of appressorium development in pathogenic fungi. Curr Opin Plant Biol. 26:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakulkoo W, Osés-Ruiz M, Garcia EO, Soanes DM, Littlejohn GR, Hacker C, Correia A, Valent B, Talbot NJ. 2018. A single fungal MAP kinase controls plant cell-to-cell invasion by the rice blast fungus. Science. 359:1399–1403. [DOI] [PubMed] [Google Scholar]
- Saunders DGO, Aves SJ, Talbot NJ. 2010a. Cell cycle–mediated regulation of plant infection by the rice blast fungus. Plant Cell. 22:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders DGO, Dagdas YF, Talbot NJ. 2010b. Spatial uncoupling of mitosis and cytokinesis during appressorium-mediated plant infection by the rice blast fungus Magnaporthe oryzae. Plant Cell. 22:2417–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazer S, Lynch M, Needleman D. 2014. Deciphering the evolutionary history of open and closed mitosis. Curr Biol. 24:R1099–R1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K-F, Osmani AH, Govindaraghavan M, Osmani SA, Solomon MJ. 2014. Mitotic regulation of fungal cell-to-cell connectivity through septal pores involves the NIMA kinase. Mol Biol Cell. 25:763–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman EN, Jones K, Jenkinson CB, Kim DW, Zhu J, Khang CH. 2017. Nuclear and structural dynamics during the establishment of a specialized effector-secreting cell by Magnaporthe oryzae in living rice cells. BMC Cell Biol. 18:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G, Schuster M, Theisen U, Kilaru S, Forge A, Martin-Urdiroz M. 2012. Motor-driven motility of fungal nuclear pores organizes chromosomes and fosters nucleocytoplasmic transport. J Cell Biol. 198:343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube A, Weber I, Steinberg G. 2005. A novel mechanism of nuclear envelope break-down in a fungus: nuclear migration strips off the envelope. EMBO J. 24:1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suelmann R, Sievers N, Fischer R. 1997. Nuclear traffic in fungal hyphae: in vivo study of nuclear migration and positioning in Aspergillus nidulans. Mol Microbiol. 25:757–769. [DOI] [PubMed] [Google Scholar]
- Takeshita N. 2016. Coordinated process of polarized growth in filamentous fungi. Biosci Biotechnol Biochem. 80:1693–1699. [DOI] [PubMed] [Google Scholar]
- Veith D, Scherr N, Efimov VP, Fischer R. 2005. Role of the spindle-pole-body protein ApsB and the cortex protein ApsA in microtubule organization and nuclear migration in Aspergillus nidulans. J Cell Sci. 118:3705–3716. [DOI] [PubMed] [Google Scholar]
- Veneault-Fourrey C, Barooah M, Egan M, Wakley G, Talbot NJ. 2006. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science. 312:580–583. [DOI] [PubMed] [Google Scholar]
- Xiang X. 2018. Nuclear movement in fungi. Semin Cell Dev Biol. 10.1016/j.semcdb.2017.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam C, He Y, Zhang D, Chiam K-H, Oliferenko S. 2011. Divergent strategies for controlling the nuclear membrane satisfy geometric constraints during nuclear division. Curr Biol. 21:1314–1319. [DOI] [PubMed] [Google Scholar]


