Abstract
Molecular and cellular heterogeneity are phenomena that are revolutionizing oncology research and becoming critical to the idea of personalized medicine. Recent comprehensive molecular profiling has identified molecular subtypes of gastric cancer (GC) and linked them to clinical information. Moreover, GC stem cells (gCSCs) have been identified and found to be responsible for GC initiation and progression, Helicobacter pylori oncogenic action and therapy resistance. Addressing molecular heterogeneity is critical for achieving an optimal therapeutic approach against GC as well as targeting gCSCs. In this review, we outline the implications of molecular and cellular heterogeneity in the treatment of GC and we summarize the clinical impact of the most important regulators of gCSCs.
Keywords: cellular heterogeneity, gastric cancer, gastric cancer stem cells, molecular heterogeneity, personalized medicine
Gastric cancer
Gastric cancer (GC) is the fourth most common cancer and the third leading cause of cancer-related mortality worldwide. The 5-year survival rate is less than 5% in advanced unresectable or metastatic disease,1 a stage observed in around 80% of patients at diagnosis.2 GC is a complex disease influenced by a range of environmental and genetic factors. Among the former, which include smoking and a high-salt diet, infection with Helicobacter pylori (H. pylori) is a major driver that promotes chronic inflammation in the gastric epithelium and the sequential histological alterations that lead to gastric carcinoma.3 In this process, genetic and epigenetic alterations occur and accumulate, such as mutations in the APC, TP53 and KRAS genes, or the phenomenon of DNA hypermethylation.2 Regarding the therapeutic options, surgical resection along with adjuvant or neoadjuvant radiotherapy and chemotherapy based on cisplatin, 5-fluorouracil, taxanes or irinotecan is the most potentially curative treatment for GC. However, despite the growing knowledge and the advances in drug development, GC presents a poor outcome due to late diagnosis and the extremely high heterogeneity exhibited both within tumors and between patients. This heterogeneity makes the choice of therapy difficult and underlines the need for novel markers for patient stratification, as well as the need for therapies capable of addressing genetic, molecular and cellular heterogeneity within tumors.
GC classifications
Classically, GC has been classified according to Lauren’s histological criteria into intestinal (50%), diffuse (33%) or mixed/unclassified (17%).4 Intestinal GC is characterized by tumor cells arranged in tubular or glandular formations, which are often associated with intestinal metaplasia, whilst diffuse GC lacks intercellular junctions, with cancer cells infiltrating the stroma. The diffuse subtype is associated with younger patients and a poorer prognosis than the intestinal type.5 In 2010, the World Health Organization (WHO) classified GC histologically, according to the predominant pattern, into papillary, tubular, mucinous and poorly cohesive. The papillary type is characterized by elongated finger-like structures lined by cylindrical or cuboidal cells supported by fibro vascular connective tissue cores. This subtype is frequently associated with liver metastasis and extensive lymph node involvement.6 In the tubular pattern, cells are arranged in branching tube-like epithelial structures, sometimes accompanied by acinar structures. The mucinous subtype contains malignant glandular cells and may harbor scattered signet-ring cells. Finally, poorly cohesive carcinomas are composed of scattered cancer cells, appearing as isolated cells or in small clusters. In some cases, tumors are mainly composed of signet-ring cells and others contain cells that resemble histiocytes, lymphocytes or plasma cells. Poorly cohesive carcinomas correspond to the diffuse type described by Lauren, while tubular and papillary carcinomas approximately correspond to the intestinal type.
Histological classifications per se are not sufficient to explain the high complexity of GC. In recent years, there has been outstanding progress in the elucidation of the genomic landscape of GC due to technical advances and the efforts of international research consortiums such as the Cancer Genome Atlas (TCGA) Research Network7 and the Asian Cancer Research Group (ACRG).8 This research has demonstrated that GC exhibit a broad plethora of gene mutations and amplifications, diverse DNA methylation profiles, and differences in the activation or inactivation of particular signaling cascades. In response to these findings, novel classifications of GC have been proposed, with distinct subtypes based on molecular alterations (Figure 1).
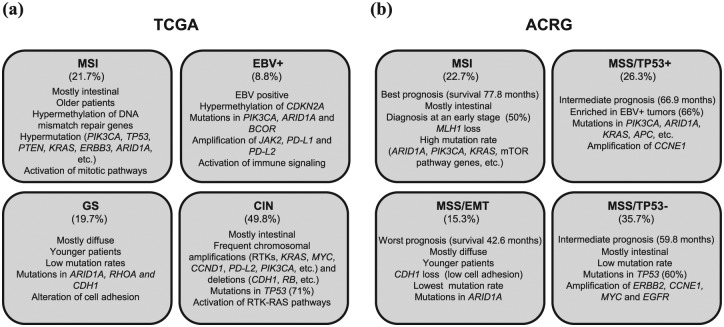
Figure 1.
Characteristics of the Cancer Genome Atlas (TCGA) Research Network and the Asian Cancer Research Group (ACRG) gastric cancer cohorts. A summary with the different proposed subtypes is presented for each cohort. (a) TCGA subtypes: MSI: tumors with microsatellite instability; EBV+: tumors positive for Epstein-Barr virus; GS: genomically stable tumors; CIN: tumors with chromosome instability. (b) ACRG subtypes: MSI: tumors with microsatellite instability; MSS/TP53+: stable tumors with active TP53; MSS/TP53–: stable tumors with inactive TP53, MSS/EMT: stable tumors expressing an EMT signature. The clinical characteristics and main genetic and molecular alterations are listed for each subtype.
From analysis of information gathered on a large number of patients (n = 295) from across the world and unsupervised and integrative clustering of molecular data, the TCGA Research Network has identified four GC subtypes: tumors positive for Epstein-Barr virus (EBV+), tumors showing microsatellite instability (MSI), genomically stable (GS) tumors and cases exhibiting chromosomal instability (CIN) [Figure 1(a)]. Patients with the MSI subtype (21.7%) generally have intestinal type tumors and are diagnosed at older ages. This subtype is associated with methylation of DNA mismatch repair genes (including specifically MLH1) and a high incidence of mutations in PIK3CA, ERBB3, RNF43, PTEN, TP53, KRAS or ARID1A. The EBV+ group represents 8.8% of cases and is characterized by DNA hypermethylation (CDKN2A being hypermethylated in all cases but MLH1 in none), mutations in PIK3CA, ARID1A and BCOR, and amplification of JAK2, CD274 (PD-L1) and PDCD1LG2 (PD-L2). According to the degree of aneuploidy, the rest of the tumors were classified as GS or exhibiting CIN. GS cases (19.7%) are mainly diffuse and diagnosed in younger patients (median age of 59 years). They have low mutation rates, ARID1A, RHOA and CDH1 being the most frequently mutated genes. The CIN group represents almost half of cases (49.8%) and is characterized by frequent copy number alterations. Amplification of genes encoding receptor tyrosine kinases (EGFR, ERBB2, ERBB3, FGFR2 and MET) is one of the distinctive aspects of these tumors, which also show amplification of transcription factors (MYC and GATA4, among others), cell-cycle regulators (CDK6, CCNE1 and CCND1) and other genes such as PDCD1LG2 (PD-L2) or PIK3CA. Chromosomal deletions affecting CDH1, CTNNA1 and RB1, and mutations in TP53 (71%) are also frequent in this group.7 Interestingly, the integration of molecular data with data related to known signaling pathways revealed a marked immune cell signaling in the EBV+ subtype, prominent alteration of cell adhesion in the GS subtype, receptor tyrosine kinase (RTK)/RAS signaling pathway activation in the CIN subtype and activation of mitotic pathways in the MSI subtype.7
The ACRG analyzed samples from 300 Korean patients and classified GC according to defined genetic signatures, the status of TP53 activation and the MSI condition.8 Thereby, the group identified four molecular subtypes: tumors showing MSI, microsatellite stable TP53 active (MSS/TP53+), microsatellite stable TP53 inactive (MSS/TP53–) and microsatellite stable expressing an EMT signature cases (MSS/EMT) [Figure 1(b)]. Notably, these subtypes are associated with different survival rates and recurrence patterns. Specifically, the MSI subtype has the best prognosis (mean survival of 77.8 months) and the lowest tendency to recur, followed by the MSS/TP53+ and MSS/TP53– subtypes, with intermediate prognosis (mean survival of 66.9 and 59.8 months respectively), while the poorest outcome and highest recurrence rate (63%) are associated with the MSS/EMT subtype (mean survival of 42.6 months). MSI tumors (22.7%) are diagnosed at an early stage (I/II), are commonly intestinal (60% of them), and show loss of MLH1 and a high frequency of mutations in ARID1A, KRAS, PIK3CA, ALK and genes involved in the phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) pathway. The MSS/TP53+ subtype (26.3%) is more enriched in EBV+ cases (66%) than the others, and has a higher prevalence of mutations than the MSS/TP53– subtype in genes such as APC, ARID1A, KRAS, PIK3CA and SMAD4. The most frequent gene amplification in these cases affect CCNE1. The MSS/TP53– subtype (35.7%) is mainly intestinal and carries mutations in TP53, and has a relatively low frequency of mutations affecting other genes. This subgroup also has amplification of ERBB2, CCNE1, MYC and EGFR genes. MSS/EMT tumors (15.3%) are predominantly diffuse (>80%) and diagnosed at a younger age (median of 53 years versus 64–66 years in the other subtypes). This subtype presents low cell adhesion due to the loss of CDH1, and exhibits the fewest mutations, ARID1A being among the most frequently mutated genes (13.9%). Notably, this classification has been shown to be applicable to other independent large cohorts, in which it also segregates cases into the same four defined subgroups and the association with prognosis remains.8
TCGA and ACRG classifications exhibit some differences that could be attributed to the application of different approaches and technological platforms, as well as differences in the ethnicity of the patients, which are mainly from Korea in the study of the ACRG and from USA and Western Europe in the TCGA analysis.7,8 Moreover, the histological diffuse type is more represented in the ACRG cohort (45% in ACRG and 24% in TCGA). Nonetheless, they also present similarities such as both consortia identified a MSI subtype, with MLH1 hypermethylation and high mutation frequency. EBV and MSS/TP53+ subtypes also share similarities since MSS/TP53+ cases are frequently positive for EBV infection and some of their frequently mutated genes such as PIK3CA and ARID1A are common. However, the alteration of these genes does not define these subgroups of patients, since they have been described in several of the other subtypes of tumors. There are also analogies between GS and MSS/EMT subtypes, which affect younger patients, are mostly diffuse and present low intercellular adhesion. Finally, both CIN and MSS/TP53– subtypes are mostly intestinal, present mutations in TP53 and exhibit amplification of EGFR family members.
GC molecular heterogeneity and therapy
Regarding GC therapeutic options, surgical resection along with adjuvant or neoadjuvant radio-therapy and chemotherapy based on cisplatin, 5-fluorouracil, taxanes or irinotecan is the first-line and most potentially curative treatment for GC. Patients with advanced disease receive palliative chemotherapy and those whose tumors overexpress the human epidermal growth factor receptor 2 (HER2) receive trastuzumab (Herceptin, Roche, Germany) as first-line treatment. Another targeted therapy approved for the treatment of refractory advanced GC is ramucirumab (Cyramza, Lilly, USA), an antibody directed against the vascular endothelial growth factor receptor 2 (VEGFR2), that inhibits angiogenesis and prolongs survival alone or in combination with paclitaxel.9 However, despite the advances in drug development, GC still presents a poor outcome.
As indicated in the previous section, GC heterogeneity is manifested in marked differences in disease aggressiveness and treatment outcome. The identification of the different molecular subtypes of GC represents an advance towards the goal of personalized medicine. Nevertheless, in current clinical practice, the choice of therapeutic strategy against GC still does not consider this molecular heterogeneity regularly and is mostly based on tumor stage (Figure 2). Thus, heterogeneity is likely to explain, at least in part, the dismal results obtained in many clinical trials, since the criteria for selection of patients in most cases are not based on molecular information. It is therefore reasonable to surmise that some agents that have not provided significant benefits in nonstratified patients with GC could be beneficial in specific subsets of patients.
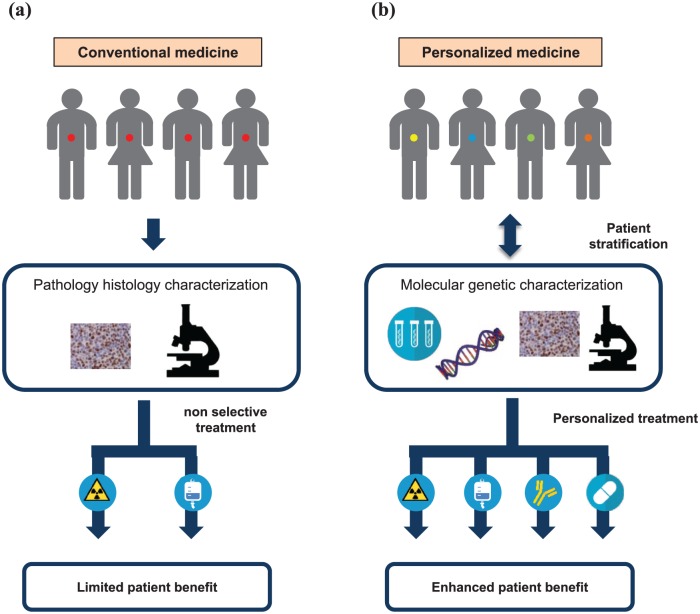
Figure 2.
Impact of molecular heterogeneity and personalized treatment in gastric cancer (GC). (a) Historically, patients with GC have been treated uniformly, and this has been associated with therapeutic effects in a limited percentage of patients, as it does not take into account the high molecular heterogeneity present among patents with GC. (b) Screening to identify theranostic biomarkers is a necessary condition for personalized medicine approaches. In this case, the identification of specific biomarkers would allow patient stratification and subsequently personalized treatment, ensuring that each subgroup or individual receives the most appropriate and effective treatment or drug. This approach might significantly increase the therapeutic effects in patients.
In particular, based on the molecular studies referred to above, drugs directed against RTKs such as cetuximab, rilotumumab or dovitinib could represent suitable therapies for certain CIN cases, which overexpress their targets. In relation to this, some trials are underway testing an antibody against FGFR-2 in FGFR-2-amplified gastric cancers [ClinicalTrials.gov identifier: NCT02318329] and the antibody nimotuzumab against EGFR is being tested in combination with irinotecan in patients with high EGFR expression [ClinicalTrials.gov identifier: NCT01813253].9 Similarly, the mTOR inhibitor everolimus or other analogues could be effective in subsets of patients with MSI subtype, since they frequently harbor activating mutations in genes involved in the mTOR pathway. Furthermore, it is also plausible that those patients harboring MSI and CIN tumors with alterations in PIK3CA or PTEN or genes encoding RTKs respond to PI3K inhibitors. Indeed, nowadays a trial is evaluating the efficacy of the inhibitor of PI3K-β GSK2636771 in patients with advanced gastric carcinomas deficient in the Phosphatase and tensin homolog (PTEN) tumor suppressor gene [ClinicalTrials.gov identifier: NCT02615730].10 In relation to PI3K, but using it as a marker, another clinical trial is being developed to test the efficacy of the AKT inhibitor AZD5363 in combination with paclitaxel for the treatment of advanced gastric carcinoma with PIK3CA mutation or amplification [ClinicalTrials.gov identifier: NCT02451956].10 Also in relation to AKT as target, a recent study has revealed that the loss of ARID1A expression in GC cells due to mutations leads to the activation of AKT, suggesting that the mutational status of ARID1A in GCs may define the response of patients to therapies with AKT inhibitors.11 ARID1A mutation is a very widespread event in GC, but it is especially frequent in the MSI subtype,12 in which other alterations coexist that lead to the activation of AKT as mutations in PIK3CA, PTEN or ERBB3. Thus, it would be worth analyzing the response of patients with GC MSI to the inhibition of AKT, and even in combination with PI3K inhibitors. As we have mentioned, trastuzumab is being used for patients with advanced GC whose biopsies express HER2. However, trastuzumab offers very limited benefits in most patients and the effectiveness does not last long.13 Interestingly, ERBB2 amplification is present in some patients with the MSS/TP53– subtype (17.4% of them), and the TP53 mutation is a condition that predicts benefit from trastuzumab in other cancers.14 This could also be the case in GC and it would be worthwhile investigating whether patients with the MSS/TP53– subtype and HER2 overexpression are especially responsive to treatment with trastuzumab. Nonetheless, the definition of HER2+ GC tumors remains controversial, probably due to the lack of an unified criterion,9 and it is necessary to make an effort in this sense to stratify patients more accurately.
Immunotherapy has been successfully implemented in the treatment of some types of cancer and represents a promising strategy, which is revolutionizing oncology research.15 In GC, the antibody nivolumab (Opdivo, Bristol-Myers Squibb, USA), which binds to the programmed cell death protein 1 (PD-1) and disrupts its interaction with its ligands PD-L1 and PD-L2, enhancing the antitumor activity of T lymphocytes, was approved in Japan in 2017 as third-line therapy for its benefit in patients with unresectable or recurrent gastric carcinoma treated with at least two prior lines of chemotherapy.16 Similarly, another clinical trial has led to the US Food and Drug Administration approval of the anti-PD-L1 antibody pembrolizumab (Keytruda, Merck & Co., USA), for the treatment of patients with PD-L1+ recurrent or advanced gastric adenocarcinoma with disease progression after two or more lines of therapy.17 Remarkably, immunotherapy could also be beneficial as first line and it could be especially suitable for particular subgroups of patients. Notably, if we put our attention on immunotherapy targets and the GC classifications presented above, the TCGA study revealed amplification of PD-L1 or PD-L2 in the EBV+ and CIN subtypes. Thus, it is conceivable that a relatively high proportion of patients with EBV+ or CIN tumors would respond to immune checkpoint blockade. Further, MSI cases could also respond to this type of therapy because they present mismatch-repair deficiency, a condition that in other cancers such as colorectal predicts benefit from immune checkpoint blockade with pembrolizumab.18 In support of the use of immunotherapy in GC, a recent meta-analysis focused on PD-L1 in GC has shown that PD-L1 overexpression is associated with poor prognosis and is significantly related to EBV infection and MSI status, reinforcing the interest of this target in both subtypes.19
GC heterogeneity at the cellular level
Intratumor heterogeneity in GC is also evident at the cellular level (Figure 3). GCs have a particularly important small subpopulation of cells that displays characteristics similar to stem cells, including unlimited self-renewal and multilineage differentiation potential. These cells, called GC stem cells (gCSCs), were first isolated from human GC biopsies in 2007,20 yet their origin remains somewhat unclear. Cancer is the result of the accumulation of multiple genetic alterations that only occurs and perpetuates in long-lived and self-renewing cells such as adult stem cells.21 In accordance with this notion, studies in mice have revealed that the induction of genetic or epigenetic events specifically in gastrointestinal stem cells lead to the development of GC.22,23 Moreover, this malignant transformation of gastric stem cells into gCSCs also happens as a result of H. pylori infection.24,25 Other origins for gCSCs have been proposed, such as bone-marrow-derived cells recruited to the stomach in response to Helicobacter infection26 or cells derived from the dedifferentiation of gastric epithelial cells.27
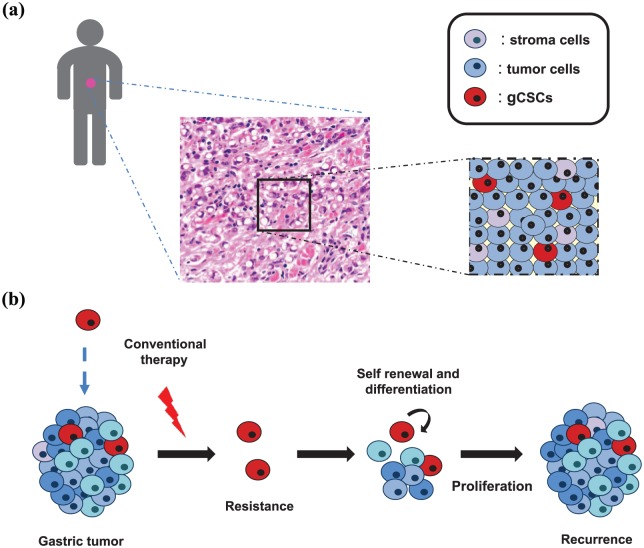
Figure 3.
Proper management of gastric cancer (GC) demands consideration of the intratumoral cellular heterogeneity. (a) Gastric tumors are complex entities, highly heterogeneous at the cellular level, composed of different populations of tumor and stromal cells and containing GC stem cells (gCSCs; in red), a minority undifferentiated, self-renewing and quiescent cell population. (b) These characteristics suggest that gCSCs are responsible for cancer initiation, resistance to therapy and recurrence. Consequently, identifying gCSC biomarkers and regulators is critical for the treatment of GC and should be taken into account in personalized medicine approaches.
In addition to tumor origin, growing evidence indicates that gCSCs are responsible for long-term tumor maintenance, chemotherapy resistance, recurrence and metastasis.28–34 It is well known that conventional chemotherapy does not take into account intratumor heterogeneity at the cellular level and is most effective in proliferative cells. Hence, it does not target cancer stem cells (CSCs). In fact, subpopulations of cells within tumors expressing stem cell markers exhibit resistance to a broad spectrum of chemotherapeutic agents and radiation.28–31 This resistance is due in part to their quiescent status and their capacity of self renewal. In line with this, in patients with GC, residual tumor tissue after chemotherapy treatment is enriched in the expression of postulated gCSC markers.32 In addition, gCSCs have been implicated in the process of metastasis in GC. The expression of gCSC markers in primary GC is associated with an increased risk of metastasis and a dismal prognosis.33 Furthermore, patients with GC and circulating tumor cells that express CSC markers have earlier recurrence and are more likely to develop metastasis.34,35
All these findings, taken together, highlight the importance of identifying the regulators of gCSCs for their use as biomarkers for patient stratification as well as molecular targets. Moreover, it would be important to link their expression to the established clinical and pathological prognostic factors, the recently identified genetic mutations and molecular alterations that are drivers and passengers of GC progression and the aforementioned novel classifications. Classically there has been great interest in the search for surface markers that are differentially expressed in CSCs given the possibility of identifying, isolating and targeting this cell population through the use of antibodies. However, other intracellular proteins such as transcription factors or enzymes are also relevant in the biology of CSCs and may constitute suitable molecular GC biomarkers and also therapeutic targets. In this review, we summarize the current knowledge regarding the impact of the most relevant regulators of gCSCs in GC pathobiology.
Regulators of gCSCs
CD44
CD44 is a trans membrane glycoprotein that binds to hyaluronic acid (HA) in the extracellular matrix36 and also interacts with osteopontin, collagens or matrix metalloproteinases (MMPs). It is a fetal and adult hematopoietic stem cell regulator that participates in cell–cell interactions, cell adhesion and migration, and is involved in processes such as lymphocyte activation and homing.37 The standard form of CD44 (CD44s) comprises 10 exons and alternative splicing results in many distinct variants (CD44v).38 The role of CD44 as a robust and widespread CSC marker has been described elsewhere.39 In GC, Takaishi and collaborators originally found that CD44+ cells isolated from GC cell lines had self-renewal and tumorigenic potential when inoculated into immunodeficient mice. Moreover, CD44 silencing abrogated these stem cell properties, this being the first gCSC biomarker proposed.28 Accordingly, enhanced chemo resistance and invasiveness for CD44+ cells have also been shown in GC cell lines.30 In line with the role of CD44 as a biomarker of gCSCs, the analysis of gastric tissue samples from patients suggests that the emergence of gCSCs induced by H. pylori infection of gastric mucosa may rely on CD44 induction.40 Nevertheless, some studies have not found CSC characteristics in the subpopulations of CD44+ cells isolated from patient-derived xenografts,41,42 and it has been suggested that some CD44 variants could be more relevant for gCSCs than CD44s.43
Regarding the relevance of CD44 to clinical practice, CD44 expression in gastric tumors is related to adverse clinical and pathological features. In particular, high CD44 expression is associated with larger tumor size,44 low grade of differentiation,45,46 tumor relapse,46 lymph node invasion,44,47 distant metastasis44,46 and reduced survival.30,44–46 Congruently, the frequency of circulating CD44+ tumor cells in patients with GC correlates with disease stage.48 Furthermore, the number of CD44 variants present in tumors is related to prognosis, suggesting that different isoforms may have different functions in GC.43
All these findings taken together reveal the importance of CD44 in GC and suggest that it could be used as GC biomarker and that its inhibition could represent a useful therapy. Interestingly, the combination of CD44 expression together with the expression of EMT markers predicts early recurrence of GC after surgery.49 Therefore, it is feasible that CD44 targeting could have a special relevance in the MSS/EMT subtype identified by the ACRG. However, CD44 binding to HA activates RHOA in a process that promotes the progression of some types of cancer, such as breast cancer and head and neck squamous cell carcinoma.50 RHOA mutations are found in GC, particularly in patients with the GS subtype, and these mutations are predicted to confer gain of function.7,51,52 Moreover, increased RHOA activity correlated with poorer overall survival in patients with diffuse GC,53 which is the predominant histologic type found in the GS subtype. Hence, CD44 activity may be most relevant in the molecular context of the gastric GS subgroup. In relation to therapy, it has been observed that the inhibition of the Sonic Hedgehog (SHH) pathway, specifically the inhibition of SMO with vismodegib (Genentech, USA), reverses in vitro the chemoresistance of CD44+ CG cells.30 Moreover, in patients with GC, high CD44 expression is associated with decreased survival in response to chemotherapy alone, whereas high CD44 expression is associated with improved survival in those patients receiving chemotherapy plus vismodegib.30 This fact encourages the use of SMO inhibitors in patients with GC and high CD44 expression. The relevance of CD44 in the different identified GC molecular subtypes is not known, but since CD44 activates RHOA, it could be more relevant in cases with mutations in RHOA, and so it is conceivable that vismodegib can benefit patients with GS tumors.
CD133
CD133 is a trans membrane glycoprotein present in embryonic epithelial structures that functions as an organizer of the plasma membrane topology and lipid composition.54 It has been widely found to be a regulator of CSCs in a variety of cancers.55 Studies performed in GC cell lines demonstrate that CD133+ cells present a CSC phenotype, since they are more tumorigenic and chemoresistant, and exhibit greater migration and invasion capacities than CD133– cells.56–58 However, other studies using GC cell lines or cells derived from resected gastric cancer biopsies have also shown that CD133 expression is not indispensable for GC cells to exhibit enhanced tumorigenicity in vivo or high capacity to form spheroid colonies.28,41,59 Notably, CD133 expression is higher in GC tissue than non-neoplastic gastric mucosa in patient samples.45,60 Further, expression of this glycoprotein is associated with adverse clinical and pathological features like venous invasion, larger tumor size or higher grade,56,60,61 and it predicts reduced overall and disease-free survival,45,56,60,61 underlining its relevance to clinical practice. Regarding GC subtypes, CD133 expression is significantly associated with intestinal gastric cases,61 but in the ACRG and TCGA analyzes, overexpression of CD133 has not been associated with any specific molecular subtype. Regarding the options to attack CD133+ cells in cancer, a recent phase I clinical trial has demonstrated the feasibility, safety and efficacy of autologous chimeric antigen receptor modified T cells directed against CD133 (CART-133) in patients with diverse advanced or refractory and metastatic solid cancers (hepatocellular, pancreatic and colorectal carcinomas) [ClinicalTrials.gov identifier: NCT02541370].62 Thus, despite more trials being needed, in the future, immunotherapy with anti-CD133 CAR-modified T cells could be implemented in the clinics to target gCSCs in GC.
LGR5
LGR5 is a receptor for R-spondins that is part of the WNT signaling complex63 and also a target gene of this pathway.64 Lgr5+ stem cells are the cells of origin of intestinal and colorectal cancer,65,66 and in the stomach, increasing evidence suggests that LGR5 could be a marker for stem cells and gCSCs. Further, Lgr5 expression is almost restricted to a subset of cells located at the base of the pyloric glands in mice, a distribution that is in line with the area of origin of GC in humans.65 Through in vivo lineage tracing experiments, Barker and colleagues identified that Lgr5+ cells are self renewing, multipotent and are responsible for the renewal of the gastric epithelium.22 Interestingly, the transformation of these stem cells drives gastric tumorigenesis in vivo.22,67 Further supporting its role as a gastric stem cell modulator, LGR5+ cells are expanded in GC tissues infected by H. pylori.25,68 As in mice, in the human stomach, LGR5 is expressed in the bottom of the gastric glands69 and it is particularly upregulated, among the increased expression of canonical stem regulators and EMT core genes, in GC cell line derived spheres.70 These results expand the role of LGR5 as a gastric CSC biomarker and regulator from mice to humans. Moreover, ectopic LGR5 overexpression potentiated sphere growth and the migration and chemoresistance of GC cells,70 linking LGR5 activity to the regulation of characteristic features of gCSCs. In clinical settings, independent studies have reported LGR5 overexpression in human GC samples, which progressively increases from differentiated to poorly differentiated gastric carcinomas.71,72 Furthermore, high LGR5 expression has been strongly linked to adverse clinical and pathological features such as large tumor size and lymphatic invasion,73–75 and also with earlier recurrence, metastasis and shorter survival.72,73,75 The aforementioned association between the expression of LGR5 and EMT markers in in vitro studies70 might suggest that LGR5 would be relevant in the subset of patients with MSS/EMT GC. However, this subtype of GCs are mostly histologically diffuse and LGR5 expression has been associated with the intestinal subtype,72 so that additional studies are needed to determine whether LGR5 is particularly relevant in any of the GC subtypes.
CD24
CD24 is a sialoglycoprotein physiologically expressed in developing or regenerating tissues that is expressed in hematologic malignancies and several solid cancers, including GC. Like other stem cell genes, its expression is enriched in spheres derived from GC cell lines.76 However, there are conflicting results, since Takaishi and colleagues did not find enhanced sphere-forming capacity and tumorigenicity for CD24+ populations isolated from GC cell lines.28 According to its putative role regulating CSCs, some data in mice suggest that CD24 is relevant to the procancerous effect related to Helicobacter infection,77 whilst studies in GC cultures show that it enhances cell migration, and its inhibition results in apoptosis.78 Importantly, a meta-analysis concluded that CD24 overexpression in human GC was associated with tumor depth, invasion of lymph nodes, metastasis and reduced survival.79 As is the case of CD44, functional analyses show that CD24 also exerts oncogenic signaling through the GTPase RHOA.80 Thus, CD24 expression may be especially relevant in patients with GS gastric tumors.7
CD90
CD90 is a glycoprotein anchored to the cell membrane that is a member of the immunoglobulin family of proteins. It is expressed in many cell types and is involved in processes such as cell adhesion, migration, apoptosis and T-cell activation. Interestingly, the culture of engrafted primary GC tissues under stem-selective conditions promotes enrichment in GC CD90+ cells, which exhibit tumorigenicity as single cells and have self-renewal potential, linking CD90 with gCSCs.81 Likewise, drug pressure exerted in vitro on GC cell lines increases the presence of cells with high CD90 expression and stem cell properties.82 In patients, CD90 expression is higher in tumors than normal adjacent gastric tissue.83 Mechanistically, high CD90 expression correlates with ERBB2 overexpression, while trastuzumab decreases the population of CD90+ cells in primary cancers.81 Since ERBB2 amplification is characteristic of the MSS/TP53– subgroup, these findings suggest that it would be interesting to characterize the expression of CD90 as a potential biomarker in this molecular subtype.
SOX9
SOX9 is a member of the SOX family of transcription factors, which regulate stem cell maintenance and cell fate decisions in multiple organ systems, including the gastrointestinal tract.84 It is overexpressed in a variety of human cancers, high levels of SOX9 being correlated with malignant character and self-renewal properties in colon, breast or brain cancers.85–88 In relation to gCSCs, SOX9 levels are elevated in GC cell line derived spheres, H. pylori infected cells and cisplatin-resistant cells.31 Moreover, its silencing is associated with detrimental effects on the activity of gCSCs reflected in a reduction in tumorsphere self renewal and weaker tumor-initiating potential.31 Paralleling these effects, SOX9 mediates cisplatin chemoresistance in GC cell lines.31,89 Notably, SOX9 is a critical effector of the carcinogenic action of H. pylori. The bacterium induces SOX9 expression in pretumorigenic gastric mouse cells90 and also in GC cells, especially in response to highly virulent strains.31 Notably, SOX9 is required for bacteria-induced GC cell proliferation and acquisition of stem-cell-like properties.31
Several studies have linked SOX9 expression to GC biology in clinical settings. High tumor SOX9 expression is associated with advanced TNM stages, lymph node metastasis and shorter overall patient survival.31,91 High levels of SOX9 correlate with elevated expression of CEACAM1 in human biopsies,92 a prometastatic gene associated with lymph node metastasis and advanced TNM stage in GC.93 Furthermore, elevated SOX9 expression is associated with the activation of the WNT canonical oncogenic pathway, with which it establishes a regulatory feedback loop.31 As far as SOX9 and GC molecular subtypes is concerned, SOX9 is among the genes most expressed with respect to healthy gastric tissue in patients from the TCGA and ACRG cohorts, its expression being higher in CIN, EBV+ and MSI than in the GS subtype.31
ALDH
Aldehyde dehydrogenase (ALDH) exerts a detoxifying action by the oxidation of cellular aldehydes and is also involved in retinoic acid signaling through the oxidation of retinol. High ALDH expression or enzymatic activity has been observed in CSCs.94 In GC, the fraction of ALDH+ cells present in GC cell lines, gastric human biopsies and patient-derived xenografts display self-renewal capacity, high tumorigenicity, multilineage differentiation potential and chemoresistance,29,95–97 suggesting that this may be a robust gCSC biomarker. In line with this idea, the ALDH+CD44+/CD166+ signature corresponded to the most tumorigenic phenotype among cells derived from human primary GCs.97 Finally, the ALDH-3A1 isoform of ALDH is the most overexpressed in gCSCs, and its expression at the protein level in human GC biopsies correlated with dysplasia, lymph node metastasis and tumor stage.98 These results link ALDH to the pathology of GC and future work should explore whether it might be associated with any particular GC subtype. From a therapeutic point of view, it was observed that in vitro transforming growth factor (TGF)-β decreases the cancer-initiating cell population within diffuse-type gastric carcinoma cells, wherein it downregulates ALDH1 expression.96,99 Accordingly, in human diffuse-type gastric carcinoma tissues, the expression of ALDH1 at the protein level correlated inversely with Smad3 phosphorylation as a measure of TGF-β signalling.96 Nevertheless, TGF-β exerts a relevant oncogenic activity in GC, so that the administration of TGF-β with the aim of targeting gCSCs could represent a double-edged sword.
Concluding remarks
The improvement in our understanding of the pathology of GC has greatly hastened over the last decade. The advent of sophisticated genomic tools has allowed deciphering and upgrading our understanding of the molecular pathology of GC, enabling us to redefine the disease at the molecular level. Moreover, it has been firmly established that GC exhibits significant intratumor cellular heterogeneity and plasticity, increasing our understanding of the biology of GC, suggesting novel molecular targets and proposing the re-evaluation of conventional therapeutic strategies. The development of molecularly targeted therapies, coupled with robust and accurate biomarkers and improved diagnostic criteria, holds the promise of delivering a new era in GC treatment. In this sense, a big effort is being done in the field of bioinformatics in the integration of molecular information available (gene expression, mutations, methylation, copy number alterations, etc.) oriented to understand the mechanism of drug response for the improvement of the treatment. Nevertheless, in this context it is important to underline that despite the fact that numerous clinical trials are ongoing in GC, most of them are still not based on markers,9 an aspect that undoubtedly underestimates the potential of many therapeutic agents.
It is interesting to note that in the search for response markers, the expression of the molecular targets to which the drugs are directed does not necessarily define response. An example is the case of ramucirumab, which improves the survival of patients with advanced GC as second-line treatment,100,101 without its benefit being defined by the expression of its target VEGFR2.102 In some cases, other secondary targets also inhibited by the therapeutic agents, especially when they are chemical inhibitors, could be decisive in the response, or even molecules whose action is related to the inhibited target. In the treatment of cancer, the off-target compensatory effects cannot be ignored. These compensatory effects can activate redundant pathways, counteracting in this way the effect of the drug. For this reason, targeting simultaneously different molecular pathways (horizontal inhibition) might represent a good strategy that has been extensively evaluated and implemented in multiple types of cancer. However, it is also important to take into account that after a treatment, heterogeneous resistance mechanisms emerge and it has been shown that resistance in some cases involves the reactivation of the same pathway that is inhibited by the therapeutic agent. For this reason, inhibiting multiple nodes of the same pathway (vertical inhibition) also represents a therapeutic strategy that requires attention and further investigation.
It is clear that the effective treatment of GC must take into account the molecular heterogeneity and be well defined according to molecular markers defining response. Nonetheless, it is also essential to target the populations of gCSCs, because given their unique characteristics of quiescence, self renewal and so on, they are resistant to conventional therapies and responsible for many of the phenomena of recurrence. Regarding the attack of gCSCs, this also demands the identification of optimal molecular targets and in this work we have presented different molecules that are relevant in these cells and could be exploited in the treatment. One proposed method of gCSC-specific treatment is the use of antibodies conjugated to cytotoxic compounds or the so-called functional antibodies, whose binding inhibits the function of the target. These approaches have shown good results in preclinical trials in animal models of various types of cancer,103 but some drawbacks have to be taken into account since some of the markers (antigens) are also expressed in adult stem cells and even in normal tissues. Indeed, CD44 is expressed in most epithelial and lymphatic tissues and also in some populations of adult stem cells. For their part, CD133 and CD24 are rarely expressed in normal tissues but are present in some adult stem cells, such as hematopoietic or intestinal stem cells respectively.104 Another therapeutic possibility is the production of T cells which bind to CSC-specific antigens (CAR T-cell therapy). Although this strategy is not exempt from the problems related to the ubiquity of the molecules used as targets, currently two CAR T-cell therapies have been approved for the treatment of lymphomas and the field is rapidly evolving.
We have ahead of us a very interesting horizon in the fight against GC in which correct integration and interpretation of the genomic data, precise design of new clinical trials and new opportunities for targeting gCSCs will lead to improved quality of life and increased survival of patients with GC.
Footnotes
Funding: E.C-G. is a recipient of a Stop Fuga de Cerebros postdoctoral fellowship. M.G-P. is a recipient of a predoctoral fellowship from the University of the Basque Country (15/245). The laboratory of A.M. is supported by grants from the Carlos III Health Institute and European Regional Development Fund (PI13/02277, CP16/00039, PI16/01580, DTS16/0184), Department of Health and Industry of the Basque Government, Provincial Government of Gipuzkoa and the European Union (Marie Curie CIG 2012/712404 and REFBIO).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Estefania Carrasco-Garcia, Cellular Oncology Group, Biodonostia Health Research Institute, Gipuzkoa, Spain CIBER de Fragilidad y Envejecimiento Saludable (CIBERfes), Madrid, Spain.
Mikel García-Puga, Cellular Oncology Group, Biodonostia Health Research Institute, San Sebastian, Spain.
Sara Arevalo, Cellular Oncology Group, Biodonostia Health Research Institute, San Sebastian, Spain.
Ander Matheu, Cellular Oncology Group, Biodonostia Health Research Institute, Paseo Dr. Beguiristain s/n, Gipuzkoa, 20014, Spain IKERBASQUE, Basque Foundation, Bilbao, Spain CIBER de Fragilidad y Envejecimiento Saludable (CIBERfes) Madrid, Spain.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Correa P. Gastric cancer: overview. Gastroenterol Clin North Am 2013; 42: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amieva M, Peek RM., Jr. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology 2016; 150: 64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 5. Petrelli F, Berenato R, Turati L, et al. Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: a systematic review and meta-analysis. J Gastrointest Oncol 2017; 8: 148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu H, Fang C, Chen L, et al. Worse prognosis in papillary, compared to tubular, early gastric carcinoma. J Cancer 2017; 8: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015; 21: 449–456. [DOI] [PubMed] [Google Scholar]
- 9. Apicella M, Corso S, Giordano S. Targeted therapies for gastric cancer: failures and hopes from clinical trials. Oncotarget 2017; 8: 57654–57669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol 2018; 15: 273–291. [DOI] [PubMed] [Google Scholar]
- 11. Lee D, Yu EJ, Ham IH, et al. AKT inhibition is an effective treatment strategy in ARID1A-deficient gastric cancer cells. Onco Targets Ther 2017; 10: 4153–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang K, Kan J, Yuen ST, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet 2011; 43: 1219–1223. [DOI] [PubMed] [Google Scholar]
- 13. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–697. [DOI] [PubMed] [Google Scholar]
- 14. Fountzilas G, Giannoulatou E, Alexopoulou Z, et al. TP53 mutations and protein immunopositivity may predict for poor outcome but also for trastuzumab benefit in patients with early breast cancer treated in the adjuvant setting. Oncotarget 2016; 7: 32731–32753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lipson EJ, Forde PM, Hammers HJ, et al. Antagonists of PD-1 and PD-L1 in Cancer Treatment. Semin Oncol 2015; 42: 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538–12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 2461–2471. [DOI] [PubMed] [Google Scholar]
- 17. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018; 4: e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gu L, Chen M, Guo D, et al. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PLoS One 2017; 12: e0182692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang YC, Wang SW, Hung HY, et al. Isolation and characterization of human gastric cell lines with stem cell phenotypes. J Gastroenterol Hepatol 2007; 22: 1460–1468. [DOI] [PubMed] [Google Scholar]
- 21. Blokzijl F, de Ligt J, Jager M, et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 2016; 538: 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010; 6: 25–36. [DOI] [PubMed] [Google Scholar]
- 23. Zhu L, Gibson P, Currle DS, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 2009; 457: 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giannakis M, Chen SL, Karam SM, et al. Helicobacter pylori evolution during progression from chronic atrophic gastritis to gastric cancer and its impact on gastric stem cells. Proc Natl Acad Sci U S A 2008; 105: 4358–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sigal M, Rothenberg ME, Logan CY, et al. Helicobacter pylori activates and expands Lgr5(+) stem cells through direct colonization of the gastric glands. Gastroenterology 2015; 148: 1392–1404 e1321. [DOI] [PubMed] [Google Scholar]
- 26. Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science 2004; 306: 1568–1571. [DOI] [PubMed] [Google Scholar]
- 27. Nam KT, Lee HJ, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 2010; 139: 2028–2037 e2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takaishi S, Okumura T, Tu S, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009; 27: 1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishikawa S, Konno M, Hamabe A, et al. Aldehyde dehydrogenase high gastric cancer stem cells are resistant to chemotherapy. Int J Oncol 2013; 42: 1437–1442. [DOI] [PubMed] [Google Scholar]
- 30. Yoon C, Park DJ, Schmidt B, et al. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin Cancer Res 2014; 20: 3974–3988. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Santos JC, Carrasco-Garcia E, Garcia-Puga M, et al. SOX9 elevation acts with canonical wnt signaling to drive gastric cancer progression. Cancer Res 2016; 76: 6735–6746. [DOI] [PubMed] [Google Scholar]
- 32. Bauer L, Langer R, Becker K, et al. Expression profiling of stem cell-related genes in neoadjuvant-treated gastric cancer: a NOTCH2, GSK3B and beta-catenin gene signature predicts survival. PLoS One 2012; 7: e44566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yiming L, Yunshan G, Bo M, et al. CD133 overexpression correlates with clinicopathological features of gastric cancer patients and its impact on survival: a systematic review and meta-analysis. Oncotarget 2015; 6: 42019–42027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li M, Zhang B, Zhang Z, et al. Stem cell-like circulating tumor cells indicate poor prognosis in gastric cancer. Biomed Res Int 2014; 2014: 981261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xia P, Song CL, Liu JF, et al. Prognostic value of circulating CD133(+) cells in patients with gastric cancer. Cell Prolif 2015; 48: 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lesley J, Hascall VC, Tammi M, et al. Hyaluronan binding by cell surface CD44. J Biol Chem 2000; 275: 26967–26975. [DOI] [PubMed] [Google Scholar]
- 37. Thorne RF, Legg JW, Isacke CM. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J Cell Sci 2004; 117: 373–380. [DOI] [PubMed] [Google Scholar]
- 38. Tolg C, Hofmann M, Herrlich P, et al. Splicing choice from ten variant exons establishes CD44 variability. Nucleic Acids Res 1993; 21: 1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morath I, Hartmann TN, Orian-Rousseau V. CD44: more than a mere stem cell marker. Int J Biochem Cell Biol 2016; 81: 166–173. [DOI] [PubMed] [Google Scholar]
- 40. Choi YJ, Kim N, Chang H, et al. Helicobacter pylori-induced epithelial-mesenchymal transition, a potential role of gastric cancer initiation and an emergence of stem cells. Carcinogenesis 2015; 36: 553–563. [DOI] [PubMed] [Google Scholar]
- 41. Rocco A, Liguori E, Pirozzi G, et al. CD133 and CD44 cell surface markers do not identify cancer stem cells in primary human gastric tumors. J Cell Physiol 2012; 227: 2686–2693. [DOI] [PubMed] [Google Scholar]
- 42. Fukamachi H, Seol HS, Shimada S, et al. CD49f(high) cells retain sphere-forming and tumor-initiating activities in human gastric tumors. PLoS One 2013; 8: e72438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kodama H, Murata S, Ishida M, et al. Prognostic impact of CD44-positive cancer stem-like cells at the invasive front of gastric cancer. Br J Cancer 2017; 116: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han Y, Lu S, Wen YG, et al. Overexpression of HOXA10 promotes gastric cancer cells proliferation and HOXA10(+)/CD44(+) is potential prognostic biomarker for gastric cancer. Eur J Cell Biol 2015; 94: 642–652. [DOI] [PubMed] [Google Scholar]
- 45. Wang T, Ong CW, Shi J, et al. Sequential expression of putative stem cell markers in gastric carcinogenesis. Br J Cancer 2011; 105: 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen S, Hou JH, Feng XY, et al. Clinicopathologic significance of putative stem cell marker, CD44 and CD133, in human gastric carcinoma. J Surg Oncol 2013; 107: 799–806. [DOI] [PubMed] [Google Scholar]
- 47. Isozaki H, Ohyama T, Mabuchi H. Expression of cell adhesion molecule CD44 and sialyl Lewis A in gastric carcinoma and colorectal carcinoma in association with hepatic metastasis. Int J Oncol 1998; 13: 935–942. [DOI] [PubMed] [Google Scholar]
- 48. Watanabe T, Okumura T, Hirano K, et al. Circulating tumor cells expressing cancer stem cell marker CD44 as a diagnostic biomarker in patients with gastric cancer. Oncol Lett 2017; 13: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu GF, Zhang WJ, Sun Q, et al. Combined epithelial-mesenchymal transition with cancer stem cell-like marker as predictors of recurrence after radical resection for gastric cancer. World J Surg Oncol 2014; 12: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol 2008; 18: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang K, Yuen ST, Xu J, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet 2014; 46: 573–582. [DOI] [PubMed] [Google Scholar]
- 52. Kakiuchi M, Nishizawa T, Ueda H, et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet 2014; 46: 583–587. [DOI] [PubMed] [Google Scholar]
- 53. Yoon C, Cho SJ, Aksoy BA, et al. Chemotherapy resistance in diffuse-type gastric adenocarcinoma is mediated by RhoA activation in cancer stem-like cells. Clin Cancer Res 2016; 22: 971–983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54. Mizrak D, Brittan M, Alison M. CD133: molecule of the moment. J Pathol 2008; 214: 3–9. [DOI] [PubMed] [Google Scholar]
- 55. Brungs D, Aghmesheh M, Vine KL, et al. Gastric cancer stem cells: evidence, potential markers, and clinical implications. J Gastroenterol 2016; 51: 313–326. [DOI] [PubMed] [Google Scholar]
- 56. Zhang X, Hua R, Wang X, et al. Identification of stem-like cells and clinical significance of candidate stem cell markers in gastric cancer. Oncotarget 2016; 7: 9815–9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Song Z, Yue W, Wei B, et al. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS One 2011; 6: e17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhu Y, Yu J, Wang S, et al. Overexpression of CD133 enhances chemoresistance to 5-fluorouracil by activating the PI3K/Akt/p70S6K pathway in gastric cancer cells. Oncol Rep 2014; 32: 2437–2444. [DOI] [PubMed] [Google Scholar]
- 59. Lau WM, Teng E, Chong HS, et al. CD44v8–10 is a cancer-specific marker for gastric cancer stem cells. Cancer Res 2014; 74: 2630–2641. [DOI] [PubMed] [Google Scholar]
- 60. Zhao P, Li Y, Lu Y. Aberrant expression of CD133 protein correlates with Ki-67 expression and is a prognostic marker in gastric adenocarcinoma. BMC Cancer 2010; 10: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee HH, Seo KJ, An CH, et al. CD133 expression is correlated with chemoresistance and early recurrence of gastric cancer. J Surg Oncol 2012; 106: 999–1004. [DOI] [PubMed] [Google Scholar]
- 62. Wang Y, Chen M, Wu Z, et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology 2018; 7: e1440169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. de Lau W, Barker N, Low TY, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 2011; 476: 293–297. [DOI] [PubMed] [Google Scholar]
- 64. Van der Flier LG, Sabates-Bellver J, Oving I, et al. The intestinal Wnt/TCF signature. Gastroenterology 2007; 132: 628–632. [DOI] [PubMed] [Google Scholar]
- 65. Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009; 457: 608–611. [DOI] [PubMed] [Google Scholar]
- 66. Takahashi H, Ishii H, Nishida N, et al. Significance of Lgr5(+ve) cancer stem cells in the colon and rectum. Ann Surg Oncol 2011; 18: 1166–1174. [DOI] [PubMed] [Google Scholar]
- 67. Li XB, Yang G, Zhu L, et al. Gastric Lgr5(+) stem cells are the cellular origin of invasive intestinal-type gastric cancer in mice. Cell Res 2016; 26: 838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Uehara T, Ma D, Yao Y, et al. H. pylori infection is associated with DNA damage of Lgr5-positive epithelial stem cells in the stomach of patients with gastric cancer. Dig Dis Sci 2013; 58: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu C, Xie Y, Gao F, et al. Lgr5 expression as stem cell marker in human gastric gland and its relatedness with other putative cancer stem cell markers. Gene 2013; 525: 18–25. [DOI] [PubMed] [Google Scholar]
- 70. Wang B, Chen Q, Cao Y, et al. LGR5 is a gastric cancer stem cell marker associated with stemness and the EMT signature genes NANOG, NANOGP8, PRRX1, TWIST1, and BMI1. PLoS One 2016; 11: e0168904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449: 1003–1007. [DOI] [PubMed] [Google Scholar]
- 72. Zheng ZX, Sun Y, Bu ZD, et al. Intestinal stem cell marker LGR5 expression during gastric carcinogenesis. World J Gastroenterol 2013; 19: 8714–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Simon E, Petke D, Boger C, et al. The spatial distribution of LGR5+ cells correlates with gastric cancer progression. PLoS One 2012; 7: e35486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yamanoi K, Fukuma M, Uchida H, et al. Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 in gastric cancer. Pathol Int 2013; 63: 13–19. [DOI] [PubMed] [Google Scholar]
- 75. Xi HQ, Cai AZ, Wu XS, et al. Leucine-rich repeat-containing G-protein-coupled receptor 5 is associated with invasion, metastasis, and could be a potential therapeutic target in human gastric cancer. Br J Cancer 2014; 110: 2011–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang X, Zou F, Deng H, et al. Characterization of sphereforming cells with stemlike properties from the gastric cancer cell lines MKN45 and SGC7901. Mol Med Rep 2014; 10: 2937–2941. [DOI] [PubMed] [Google Scholar]
- 77. Duckworth CA, Clyde D, Pritchard DM. CD24 is expressed in gastric parietal cells and regulates apoptosis and the response to Helicobacter felis infection in the murine stomach. Am J Physiol Gastrointest Liver Physiol 2012; 303: G915–926. [DOI] [PubMed] [Google Scholar]
- 78. Wang YC, Wang JL, Kong X, et al. CD24 mediates gastric carcinogenesis and promotes gastric cancer progression via STAT3 activation. Apoptosis 2014; 19: 643–656. [DOI] [PubMed] [Google Scholar]
- 79. Wu JX, Zhao YY, Wu X, et al. Clinicopathological and prognostic significance of CD24 overexpression in patients with gastric cancer: a meta-analysis. PLoS One 2014; 9: e114746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Deng W, Gu L, Li X, et al. CD24 associates with EGFR and supports EGF/EGFR signaling via RhoA in gastric cancer cells. J Transl Med 2016; 14: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jiang J, Zhang Y, Chuai S, et al. Trastuzumab (Herceptin) targets gastric cancer stem cells characterized by CD90 phenotype. Oncogene 2012; 31: 671–682. [DOI] [PubMed] [Google Scholar]
- 82. Xue Z, Yan H, Li J, et al. Identification of cancer stem cells in vincristine preconditioned SGC7901 gastric cancer cell line. J Cell Biochem 2012; 113: 302–312. [DOI] [PubMed] [Google Scholar]
- 83. Zhu GC, Gao L, He J, et al. CD90 is upregulated in gastric cancer tissues and inhibits gastric cancer cell apoptosis by modulating the expression level of SPARC protein. Oncol Rep 2015; 34: 2497–2506. [DOI] [PubMed] [Google Scholar]
- 84. Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 2013; 12: 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Matheu A, Collado M, Wise C, et al. Oncogenicity of the developmental transcription factor Sox9. Cancer Res 2012; 72: 1301–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Carrasco-Garcia E, Lopez L, Aldaz P, et al. SOX9-regulated cell plasticity in colorectal metastasis is attenuated by rapamycin. Sci Rep 2016; 6: 32350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Martin-Martin N, Piva M, Urosevic J, et al. Stratification and therapeutic potential of PML in metastatic breast cancer. Nat Commun 2016; 7: 12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Garros-Regulez L, Aldaz P, Arrizabalaga O, et al. mTOR inhibition decreases SOX2-SOX9 mediated glioma stem cell activity and temozolomide resistance. Expert Opin Ther Targets 2016; 20: 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang J, Xue X, Hong H, et al. Upregulation of microRNA-524–5p enhances the cisplatin sensitivity of gastric cancer cells by modulating proliferation and metastasis via targeting SOX9. Oncotarget 2017; 8: 574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Serizawa T, Hirata Y, Hayakawa Y, et al. Gastric metaplasia induced by Helicobacter pylori is associated with enhanced SOX9 expression via interleukin-1 signaling. Infect Immun 2015; 84: 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhou CJ, Guo JQ, Zhu KX, et al. Elevated expression of SOX9 is related with the progression of gastric carcinoma. Diagn Cytopathol 2011; 39: 105–109. [DOI] [PubMed] [Google Scholar]
- 92. Liu JN, Shang Guan YM, Qi YZ, et al. The evaluation of SOX9 expression and its relationship with carcinoembryonic antigen-related cell adhesion molecule 1 in gastric neoplastic and nonneoplastic lesions. Ann Diagn Pathol 2012; 16: 235–244. [DOI] [PubMed] [Google Scholar]
- 93. Shi JF, Xu SX, He P, et al. Expression of carcinoembryonic antigen-related cell adhesion molecule 1(CEACAM1) and its correlation with angiogenesis in gastric cancer. Pathol Res Pract 2014; 210: 473–476. [DOI] [PubMed] [Google Scholar]
- 94. Xu X, Chai S, Wang P, et al. Aldehyde dehydrogenases and cancer stem cells. Cancer Lett 2015; 369: 50–57. [DOI] [PubMed] [Google Scholar]
- 95. Zhi QM, Chen XH, Ji J, et al. Salinomycin can effectively kill ALDH(high) stem-like cells on gastric cancer. Biomed Pharmacother 2011; 65: 509–515. [DOI] [PubMed] [Google Scholar]
- 96. Katsuno Y, Ehata S, Yashiro M, et al. Coordinated expression of REG4 and aldehyde dehydrogenase 1 regulating tumourigenic capacity of diffuse-type gastric carcinoma-initiating cells is inhibited by TGF-beta. J Pathol 2012; 228: 391–404. [DOI] [PubMed] [Google Scholar]
- 97. Nguyen PH, Giraud J, Chambonnier L, et al. Characterization of biomarkers of tumorigenic and chemoresistant cancer stem cells in human gastric carcinoma. Clin Cancer Res 2017; 23: 1586–1597. [DOI] [PubMed] [Google Scholar]
- 98. Wu D, Mou YP, Chen K, et al. Aldehyde dehydrogenase 3A1 is robustly upregulated in gastric cancer stem-like cells and associated with tumorigenesis. Int J Oncol 2016; 49: 611–622. [DOI] [PubMed] [Google Scholar]
- 99. Ehata S, Johansson E, Katayama R, et al. Transforming growth factor-beta decreases the cancer-initiating cell population within diffuse-type gastric carcinoma cells. Oncogene 2011; 30: 1693–1705. [DOI] [PubMed] [Google Scholar]
- 100. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014; 383: 31–39. [DOI] [PubMed] [Google Scholar]
- 101. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014; 15: 1224–1235. [DOI] [PubMed] [Google Scholar]
- 102. Fuchs CS, Tabernero J, Tomasek J, et al. Biomarker analyses in REGARD gastric/GEJ carcinoma patients treated with VEGFR2-targeted antibody ramucirumab. Br J Cancer 2016; 115: 974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sneha S, Nagare RP, Priya SK, et al. Therapeutic antibodies against cancer stem cells: a promising approach. Cancer Immunol Immunother 2017; 66: 1383–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kim WT, Ryu CJ. Cancer stem cell surface markers on normal stem cells. BMB Rep 2017; 50: 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]