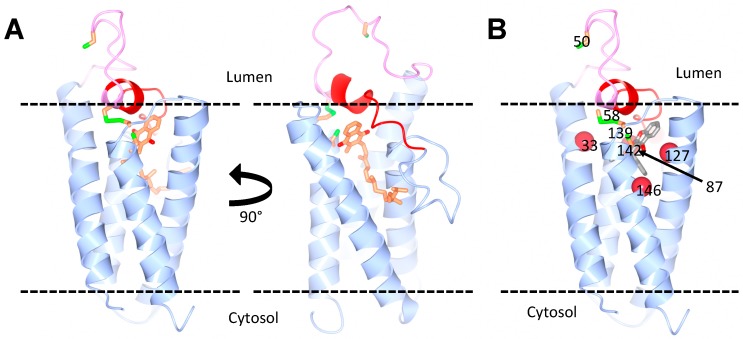
Figure 2.
Predicted structure of human VKORC1L1. (A) Homology models of VKORC1L1, bound to vitamin K, based on the structure of the bacterial VKOR homologue. The vitamin K molecule is shown in orange. Two views of the model are shown with a 90° horizontal rotation. (B) Model of VKORC1L1 bound to warfarin. The warfarin-binding pocket (grey) is surrounded by residues (red spheres) that, when mutated, cause a strong resistance in both VKOR1L1 and VKORC1 (Ala33, Asn87, Leu127 and Y146 in VKORC1L1). The cap domain (red) and the luminal loop (pink) are also highlighted. The conserved cysteines (green and orange) are shown: Cys58 and Cys139 as disulfide, and Cys50 and Cys142 as free. Courtesy of Dr. Weikai Li, Washington University in St. Louis.