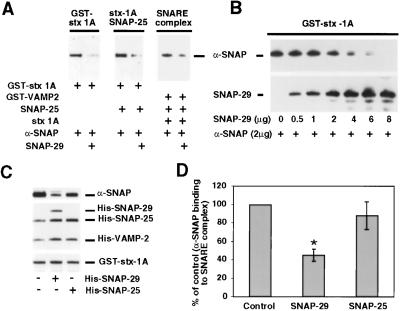
Figure 4.
SNAP-29 competes with α-SNAP for binding to SNAREs. (A) Immobilized GST–syntaxin-1A (stx-1A) or GST–stx-1A–SNAP-25 heterodimers (stx 1A-SNAP-25) and ternary SNARE complex (SNARE) on glutathione-Sepharose were incubated with 1 μg of recombinant α-SNAP in the presence (5 μg) or absence of His-SNAP-29. Bound α-SNAP was visualized by anti-α-SNAP antibody. (B) Concentration-dependent competition of SNAP-29 with α-SNAP for binding to immobilized GST–stx-1A. (C) Immobilized GST–syntaxin-1A was incubated with ≈1 μmol of His-tagged SNAP-25 and VAMP-2 to form recombinant SNARE complex in vitro, and the beads were then washed extensively. The preformed SNARE complex was then incubated with His-tagged α-SNAP (0.16 μmol) in the presence or absence of 0.8 μmol of His-SNAP-29 or His-SNAP-25 as indicated. Protein complexes were analyzed by SDS/PAGE and visualized by sequential immunoblotting. (D) The relative levels of α-SNAP binding to the SNARE complex were calculated based on a linear standard curve of α-SNAP aliquots by using National Institutes of Health image scanning. Semiquantitative analysis revealed that normalized percentage of α-SNAP binding to SNARE complex in the presence of 0.8 μmol of His-SNAP-29 (n = 5) or His-SNAP-25 (n = 3) relative to control in the absence of both SNAP-29 and SNAP-25. Data are expressed as mean ± SEM. Result marked as * (P < 0.01) was considered significantly different.