Abstract
The relationship between obesity and renal cell carcinoma (RCC) has been widely investigated. However, the effect of estrogen on this relationship in female RCC patients has not been evaluated. We conducted a case-control study to investigate the role of estrogen as a potential modifier of the association between obesity and RCC risk in Chinese women.
A total of 497 consecutive female patients with pathologically confirmed RCC, including 364 clear cell RCC (ccRCC), were enrolled. Age-matched controls were selected from cancer-free females seeking physical examination in our institution. Estrogen receptor-β (ER-β) and insulin-like growth factor (IGF)-1 receptor (IGF-1R) expression levels were detected in RCC tissues. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were estimated by logistic regression models.
We observed a positive association between overweight and RCC risk in pre-menopausal but not post-menopausal women. Similar association was also observed between overweight and ccRCC risk. Overweight pre-menopausal women had an increased risk of RCC (OR: 1.67, 95%CI: 1.01–2.76), as well as an increased risk of ccRCC (OR: 1.73, 95%CI: 1.02–2.99), after adjusting for potential confounders. IGF-1R expression levels were higher in pre-menopausal compared with post-menopausal cases (P = 0.015).
These results suggest that estrogen plays an important role in RCC etiology and may modify the association between obesity and RCC risk in women. We hypothesize that estrogen may up-regulate IGF-1R and potentiate the deleterious effects of obesity-related elevations of insulin and IGFs.
Keywords: Renal cell carcinoma, Estrogen, Obesity, Insulin-like growth factor receptor, Gender
1. Introduction
Renal cell carcinoma (RCC) is the most common kidney-derived malignancy, and accounts for 2%–3% of all adult cancers [4]. RCC has increased in incidence in recent decades, and is usually diagnosed in patients aged between 50 and 70 years [20]. Genetic background is thought to be a pivotal factor in RCC carcinogenesis; for example, approximately two-thirds of patients with clear cell RCC (ccRCC) harbor mutations in the von Hippel Lindau gene [1], while loss of chromosome 3p is also prevalent in ccRCC [15]. In contrast, environmental factors such as a Western lifestyle, including excess energy intake and minimal physical activity leading to obesity and hypertension [2,5], are also important contributory factors to RCC etiology.
The relationship between obesity and RCC risk has been widely investigated. Obesity, indicated by a high body mass index (BMI), is associated with increased long-term RCC risk [2,12] and unfavorable oncological outcomes of RCC [3]. Furthermore, obesity can promote a cascade of secondary metabolic pathologies, such as hypertension, diabetes, and dyslipidemia. These pathologies, either alone or in combination, may exacerbate the development of RCC via complex pathways involving insulin resistance, adipokines, inflammation, and other important molecular mechanisms. Many crucial signaling molecules, including the insulin-like growth factor (IGF) axis, adiponectin, and hypoxia-inducible factors, function as mediators between obesity and RCC [29,31].
Several aspects of RCC show remarkable gender differences. First, the incidence of RCC is higher in men than in women, with an approximate ratio of 2:1 [20]. Second, patients with papillary RCC are less likely to be female while chromophobe RCC patients are more likely to be female, compared with ccRCC as the major histological subtype of RCC [13]. Third, analysis of a large database found that younger female RCC patients (pre-menopausal) showed lower renal cancer-specific mortality in relation to both localized and advanced diseases compared with their male counterparts, though the sex disparities for localized and advanced RCC were diminished and even reversed, respectively, in post-menopausal women [17]. Although the reasons for these differences remain unclear, it has been supposed that sex hormones might play an important role in RCC development and in the observed disparities.
Body fat is correlated with altered insulin levels and with sex hormone secretion and storage. Previous studies reported the co-regulation of sex steroids and insulin, mainly in breast cancer [6,11]. Recent studies of the modifying effect of estrogen status on the influence of obesity on colorectal neoplasm risk [21] found that the association between obesity and colorectal cancer risk in women was limited to certain subgroups, based mostly on their estrogen status [8]. Obesity was only associated with increased colorectal adenoma risk among pre-menopausal women [27]. However, the role of estrogen status in the association between obesity and RCC risk in women has not yet been investigated. We conducted a multicenter case-control study to explore the role of estrogen as a potential modifier of the relationship between obesity and RCC risk in Chinese women. We also studied the co-regulation of estrogen and the IGF axis in RCC tissues from women to evaluate the mechanisms whereby estrogen may influence the observed association between obesity and RCC.
2. Materials and Methods
2.1. Ethics
This study was approved by the Institutional Review Board of The Affiliated Hospital of Qingdao University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all subjects prior to participation.
2.2. Study Subjects
This retrospective study included 497 consecutive female patients recruited from five regional medical centers in Eastern and Northern China (The Affiliated Hospital of Qingdao University, Songshan Hospital of Qingdao University, Fudan University Shanghai Cancer Center, The Second Affiliated Hospital of Xi'an Jiaotong University, and Baotou City Central Hospital) between January 2014 and December 2014. All patients had RCC pathologically confirmed by either renal surgery or core biopsy. Age-matched controls were selected from among cancer-free females undergoing physical examination at The Affiliated Hospital of Qingdao University during 2014. Data collection included age, height, weight, waist circumference (WC), history of hypertension and diabetes, menstrual status, use of hormone replacement therapy (HRT), histological subtype, stage at diagnosis, and Fuhrman grade. Women for whom information on menstrual status was missing or unclear were excluded from the study. A total of 445 RCC cases and 508 controls were included in the analysis.
BMI was defined as the subject's weight (kg) divided by their height (m2). BMI was categorized based on World Health Organization criteria, and a BMI ≥25 kg/m2 was considered as overweight.
2.3. Immunohistochemistry (IHC)
We obtained paraffin-embedded ccRCC specimens from the Department of Pathology for 93 ccRCC cases from The Affiliated Hospital of Qingdao University. IHC staining was carried out as described previously [30]. Briefly, fresh tissues were fixed in formalin and embedded in paraffin, then sectioned at a thickness of 5 μm. The sections were dewaxed in xylene and rinsed in alcohol and in graded alcohol/water mixtures. Hydrogen peroxide (3%) was applied to block the endogenous peroxidase activity. The sections were subsequently treated in a microwave oven twice for 6 min in citrate buffer (pH 6.0) at 600 W to undergo antigen repairing. After blocking with goat serum for 30 min, sections were incubated with primary antibodies against estrogen receptor-β (ER-β) (ab3576) (1:50) and IGF-1R (ab39398) (1:100) (Abcam, Cambridge, MA, USA) at 4 °C overnight, followed by anti-mouse/rabbit horseradish peroxidase-labeled antibody (Univ-bio, Shanghai, China) as the second antibody. ER-β and IGF-1R staining were scored as 0, 1, 2, and 3 according to the proportion of positively stained cells and the intensity of the staining, as described previously [23].
2.4. Statistical Analysis
Continuous variables were expressed as mean ± standard deviation and were compared between groups by using Student's t-tests. Categorical variables were expressed as frequencies and percentages and were compared using χ2 tests. Unconditional logistic regression analysis was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs). All statistical analyses were carried out using STATA 12.0, and two-sided P values <0.05 were considered to indicate statistical significance.
3. Results
The baseline characteristics of the 445 enrolled female RCC patients (median age, 55 years; range, 15–83 years) and 508 cancer-free female controls (median age, 53 years; range, 19–83 years) are shown in Table 1. There was no significant difference between cases and controls in terms of age, menopause, or use of HRT. However, cases were more likely to be overweight (≥25 kg/m2), have a history of hypertension or diabetes, and have a larger WC. Fuhrman grades I, II, III, and IV were observed in 22, 201, 128, and 23 patients, respectively. Information on Fuhrman grade was lacking for 71 patients (Table 1). ccRCC accounted for 364/445 (81.80%) of all cases. The clinicopathological characteristics of the 364 ccRCC patients are indicated in Table S2.
Table 1.
Clinicopathological characteristics of the 445 enrolled female RCC patients.
| Case |
Control |
P value | ||
|---|---|---|---|---|
| (n = 445) | (n = 508) | |||
| Age | 0.928 | |||
| (mean ± SD) | 53.4 ± 12.1 | 52.3 ± 12.5 | ||
| BMI | 0.020 | |||
| (n, %) | <25 | 270 (60.67) | 345 (67.91) | |
| ≥25 | 175 (39.33) | 163 (32.09) | ||
| Hypertension | 0.036 | |||
| (n, %) | yes | 169 (37.98) | 160 (31.50) | |
| no | 276 (62.02) | 348 (68.50) | ||
| Diabetes | <0.001 | |||
| (n, %) | yes | 131 (29.44) | 85 (16.73) | |
| no | 314 (70.56) | 423 (83.27) | ||
| Menopause | 0.820 | |||
| (n, %) | yes | 280 (62.92) | 316 (62.20) | |
| no | 165 (37.08) | 192 (37.80) | ||
| Use of HRT | 0.290 | |||
| (n, %) | yes | 28 (6.29) | 41 (8.07) | |
| no | 417 (93.71) | 467 (91.93) | ||
| WC | <0.001 | |||
| (n, %) | quartile1 | 94 (21.12) | 144 (28.35) | |
| quartile2 | 94 (21.12) | 143 (28.15) | ||
| quartile3 | 130 (29.21) | 111 (21.85) | ||
| quartile4 | 127 (28.54) | 110 (21.65) | ||
| Fuhrman grade | ||||
| (n, %) | I | 22 (4.94) | / | |
| II | 201 (45.17) | / | ||
| III | 128 (28.76) | / | ||
| IV | 23 (5.17) | / | ||
| missing | 71 (15.96) | / | ||
| Stage at diagnosis | ||||
| (n, %) | I | 352 (79.10) | / | |
| II | 55 (12.36) | / | ||
| III | 13 (2.92) | / | ||
| IV | 25 (5.62) | / | ||
| Pathological type | ||||
| (n, %) | ccRCC | 364 (81.80) | / | |
| papillary RCC | 18 (4.04) | / | ||
| chromophobe RCC | 40 (8.99) | / | ||
| other | 23 (5.17) | / |
BMI: Body Mass Index; WC: Waist Circumference; HRT: hormone replacement therapy; RCC: renal cell carcinoma; ccRCC: clear cell renal cell carcinoma.
We also investigated the association between obesity/overweight and RCC risk after stratification by estrogen status. Pre-menopausal, but not post-menopausal women showed a significant positive association between overweight and RCC risk (Table 2). Univariate and multivariate logistic regression models showed that overweight women with pre-menopausal status had an increased risk of RCC (OR: 1.67, 95%CI: 1.01–2.76) after adjusting for age, hypertension and diabetes, while no such positive association was observed among the subjects as a whole. Regarding WC, women in quantiles 3 and 4 were at increased risk of RCC compared with women in quantile 1 (quantile 3, OR: 1.64, 95%CI: 1.12–2.39; quantile 4, OR: 1.57, 95%CI: 1.07–2.30). In addition, there was a positive association between WC and RCC risk in pre-menopausal women (quantile 3, OR: 2.00, 95%CI: 1.09–3.66; quantile 4, OR: 2.54, 95%CI: 1.28–5.03) (Table 3). Further, we explored the association between obesity/overweight and ccRCC risk after stratification by estrogen status. As shown in Table S3 and Table S4, there was a positive association between overweight and ccRCC risk in pre-menopausal (younger) but not post-menopausal (older) women. Overweight pre-menopausal women had an increased risk of ccRCC (OR: 1.73, 95%CI: 1.02–2.99) after adjusting for potential confounders.
Table 2.
The association of obesity/overweight with RCC risk by stratification of estrogen status.
| Pre-menopausal |
Post-menopausal |
||||||
|---|---|---|---|---|---|---|---|
| Case |
Control |
P value |
Case |
Control |
P value |
||
| (n = 165) | (n = 192) | (n = 280) | (n = 316) | ||||
| BMI | 0.029 | 0.193 | |||||
| (n, %) | <25 | 111 (67.27) | 149 (77.60) | 159 (56.79) | 196 (62.03) | ||
| ≥25 | 54 (32.73) | 43 (22.40) | 121 (43.21) | 120 (37.97) | |||
| WC | |||||||
| (n, %) | quartile1 | 54 (32.73) | 80 (41.67) | 0.001 | 40 (14.29) | 64 (20.25) | 0.072 |
| quartile2 | 31 (18.78) | 58 (30.21) | 63 (22.50) | 85 (26.90) | |||
| quartile3 | 44 (26.67) | 32 (16.67) | 86 (30.71) | 79 (25.00) | |||
| quartile4 | 36 (21.82) | 22 (11.45) | 91 (32.50) | 88 (27.85) | |||
BMI: Body Mass Index; WC: Waist Circumference; RCC: renal cell carcinoma.
Table 3.
Logistic regression analysis of the association between obesity/overweight and RCC risk in overall and pre-menopausal patients.
| Overall |
Pre-menopausal |
||||
|---|---|---|---|---|---|
| OR (95% CI) | Adjusted ORa (95% CI) | OR (95% CI) | Adjusted ORa (95% CI) | ||
| BMI | <25 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥25 | 1.37 (1.05–1.79) | 1.25 (0.95–1.65) | 1.69 (1.05–2.70) | 1.67 (1.01–2.76) | |
| WC | quartile1 | 1.00 | 1.00 | 1.00 | 1.00 |
| quartile2 | 1.01 (0.70–1.46) | 0.95 (0.65–1.39) | 0.79 (0.45–1.38) | 0.80 (0.45–1.43) | |
| quartile3 | 1.79 (1.25–2.58) | 1.64 (1.12–2.39) | 2.04 (1.15–3.61) | 2.00 (1.09–3.66) | |
| quartile4 | 1.77 (1.23–2.55) | 1.57 (1.07–2.30) | 2.42 (1.29–4.57) | 2.54 (1.28–5.03) | |
BMI: Body Mass Index; WC: Waist Circumference; RCC: renal cell carcinoma.
Adjusted for age, hypertension and diabetes.
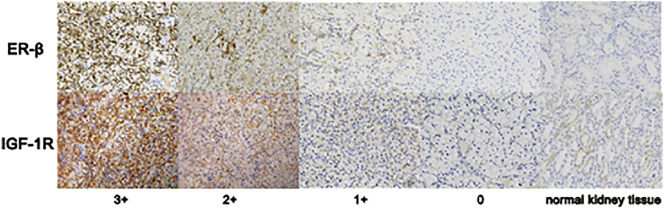
We determined the protein expression levels of ER-β and IGF-1R in 93 ccRCC specimens from female patients using IHC. The clinicopathological characteristics of these 93 patients according to estrogen status are listed in supplementary Table S1. As expected, post-menopausal patients were older than pre-menopausal ones. There were also more patients with hypertension in the post-menopausal compared with the pre-menopausal group. Fuhrman grade III + IV was more common in pre-menopausal patients. However, there was no significant difference between the groups in relation to diabetes or clinical stage (Table S1). IHC staining detected IGF-1R expression in 78.49% (73/93) of ccRCC specimens and ER-β in 22.58% (21/93) of specimens (Fig. 1). We divided patients into high- and low-expression groups for each protein, respectively, according to their expression scores: high-expression group, score 2 + 3, and low-expression group, score 0 + 1. IGF-1R expression levels were higher in pre-menopausal compared with post-menopausal ones (P = 0.015), but there was no significant difference in ER-β expression levels between the two groups.
Fig. 1.
IHC staining of ER-β and IGF-1R in ccRCC and normal kidney tissues (×400). The staining intensity was represented as followed: ER-β (upper panel) and IGF-1R (lower panel); from left to right were score 3+, 2+, 1+, 0 and normal kidney tissues respectively.
4. Discussion
As hypothesized, the results of the current study indicated that the association between obesity and RCC risk in women was modified by estrogen status, with a positive association between high BMI/WC and RCC risk in pre-menopausal, but not post-menopausal women. This apparent estrogen-based distinction in obesity-related RCC risk might be related to the IGF axis.
These findings are consistent with the results of previous studies that also found an obviously increased risk of colorectal cancer among obese pre-menopausal women, or women younger than 50 years [8], and the results of Kuper et al.'s study in ovarian cancer [10]. Moreover, our results showing a non-existent or weak association between high BMI/WC and RCC risk in post-menopausal women were also in accordance with the findings of previous studies [21,24,25]. However, studies in breast cancer patients found that obesity had less impact on cancer risk in pre-menopausal women or those using HRT [9,19]. There are several explanations for these apparent discrepancies, such as tumor heterogeneity, different ethnic backgrounds, population stratification, differences in effect sizes, and gene–environment interactions. However, despite these discrepancies, estrogen seems to play a protective role in RCC development. For example, previous studies observed an increased RCC risk after hysterectomy, suggesting that the decrease in estrogen levels after hysterectomy increased the risk of RCC [7]. Estrogen exerts its functions through binding to ERs and by subsequent regulation of the transcription and activation of downstream genes. ER-β is an ER subtype with anti-proliferative and apoptosis-inducing functions, and which has been considered as tumor suppressor [26,28]. Song et al. found that infiltrating neutrophils in RCC tissues modulated the expression of ER-β, and in turn promoted RCC cell migration [22], while another group reported that estrogen administration decreased proliferation and invasion and increased apoptosis in RCC 786-O cells [28]. However, the current study found no difference in expression levels of ER-β in RCC tissues between pre-menopausal and post-menopausal women. Since ER-β is not an estrogen target gene, we may not see any significant differences on ER-β IHC signals. However, our results suggested that estrogen status was correlated with RCC through multiple mechanisms, including a direct impact on risk as well as by modifying the effect of the association between BMI and RCC risk. Further large-scale studies are needed to clarify this issue.
Given that estrogen might influence RCC risk partly by regulating the association between obesity and RCC, we investigated the possible involvement of IGF-1R, as an important signaling molecule in obesity-related pathologies, in this process. IGF-1R expression levels were high in pre-menopausal compared with post-menopausal patients. Estrogen has been shown to up-regulate IGF-1R, leading to greater susceptibility to IGF-1 in breast cancer cells [6,16]. We therefore speculated that up-regulation of IGF-1R might increase susceptibility to obesity-induced elevations in insulin levels, and subsequent activation of the corresponding signaling pathways. Estrogen thus creates an environment in which insulin and IGFs become harmful, and potentiates the deleterious effect of IGFs. In contrast, the decline in estrogen levels after the menopause might result in a corresponding down-regulation of IGF-1R, thus reducing the impact of obesity-related pathologies. In this regard, the change in estrogen levels from the pre-menopausal to post-menopausal period might determine the direct or indirect effect of estrogen.
The ovary is the primary source of estrogen in pre-menopausal women, while adipose tissue is the main source in post-menopausal women. Elevated BMI in post-menopausal women thus results in relatively increased estrogen levels, which may be beneficial and may partly counteract the unfavorable effect of the insulin/IGF axis associated with overweight/obesity. This beneficial and counteracting effect may be negligible in pre-menopausal women because of the over-riding contribution of ovarian-derived estrogen. Nevertheless, further studies are needed to clarify the biological mechanisms underlying the complex interactions among sex hormones, obesity, and RCC risk.
One of the strengths of this study was the potential implication that obesity prevention among young women may help to protect against RCC. In addition to BMI, we also examined WC, which is thought to be more closely related to metabolic changes compared with BMI [14,18]. In addition, this was the first multicenter study focusing on the modifying effect of estrogen status on the association between obesity and RCC risk conducted in a Chinese female population. This modifying effect might help to explain how estrogen exerts its biological influence on RCC carcinogenesis. In addition to its strengths, the study also had some limitations and constraints. First, it involved a relatively small sample size and was subject to inherent biases due to its retrospective nature. Second, some potentially important information, such as aspirin and/or metformin use and the duration of HRT, was inadequate. These missing data mean that the adjustment for potential confounding factors may have been inadequate, thus weakening the conclusions. Third, we only detected IGF-1R expression in RCC tissues, and several important molecules of the IGF axis, including IGF-1, IGF-2, and IGF binding-proteins, especially those in the blood circulation, were not examined. These signaling molecules may provide more accurate information on the interactions among estrogen, obesity, and RCC. In addition, our findings also need to be explained with caution: in women, estrogen increases across pubertal development and plays important roles in organ development and function maintenance until the post-menopausal stage. Those post-menopausal women are “estrogen-positive” and their IGF axis is also elevated at their pre-menopausal ages. Hence, a possible explanation of our results is that those post-menopausal females did not develop clinically detectable RCC at their pre-menopausal ages. Further large-scale prospective studies with adequate data are therefore needed to validate the current results.
In conclusion, the results of the current study suggest that estrogen may influence the risk of RCC in women. Estrogen appears to exert a protective effect and thereby decrease RCC risk, while simultaneously intensifying the impact of obesity on RCC risk. Evaluation of this modifying effect, together with more information on the co-regulation of sex hormones, insulin, and the IGF axis, may provide deeper insights into the complex biological mechanisms involved in RCC carcinogenesis.
The following are the supplementary data related to this article.
Clinicopathological characteristics of 93 patients for whom IHC was performed.
Clinicopathological characteristics of the 364 enrolled female ccRCC patients.
The association of obesity/overweight with ccRCC risk by stratification of estrogen status.
Logistic regression analysis of the association between obesity/overweight and ccRCC risk in overall and pre-menopausal patients.
Declaration of Interests
The authors declare no competing financial interests.
Funding
This study was partly funded by the National Natural Science Foundation of China (Grant No. NSFC 81502195, NSFC 81672512) and Medicine and Health Science Technology Development Project of Shandong Province (No. 2016WS0258).
Author Contributions
Conception and design: Guiming Zhang, Lijiang Sun.
Collection and assembly of data: Fan Chao, Bo Luo, Dingwei Ye, Jun Zhao, Qiang Zhang.
Data analysis and interpretation: Xiaocheng Ma, Guiming Zhang.
IHC performance: Fan Chao.
Manuscript writing: Fan Chao, Guiming Zhang.
Final approval of manuscript: All authors.
Acknowledgments
The authors were grateful to the recruited patients and their kin. We thank Jeremy Allen, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Previous studies by Wolf and colleagues have reported that the risk of colorectal adenoma may be increased with obesity among premenopausal women but decreased among postmenopausal women, especially if they also take postmenopausal hormone use (Cancer Epidemiology, Biomarkers& Prevention). In addition, Slattery et al. also found that obesity was associated with an increased colon cancer risk among pre-menopausal but not post-menopausal women (Cancer Causes and Control).
References
- 1.Barontini M., Dahia P.L. VHL disease. Best Pract Res Clin Endocrinol Metab. 2010;24(3):401–413. doi: 10.1016/j.beem.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom A., Hsieh C.C., Lindblad P. Obesity and renal cell cancer—a quantitative review. Br J Cancer. 2001;85(7):984–990. doi: 10.1054/bjoc.2001.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Chow W.H., Devesa S.S. Contemporary epidemiology of renal cell cancer. Cancer J. 2008;14(5):288–301. doi: 10.1097/PPO.0b013e3181867628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow W.H., Gridley G., Fraumeni J.F., Jarvholm B., Jr. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med. 2000;343(18):1305–1311. doi: 10.1056/NEJM200011023431804. [DOI] [PubMed] [Google Scholar]
- 6.Dupont J., Karas M., LeRoith D. The potentiation of estrogen on insulin-like growth factor I action in MCF-7 human breast cancer cells includes cell cycle components. J Biol Chem. 2000;275(46):35893–35901. doi: 10.1074/jbc.M006741200. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein M.R., Mascitelli L. Increased risk of renal cell carcinoma after hysterectomy: possible causes and implications. Arch Intern Med. 2011;171(13):1214–1215. doi: 10.1001/archinternmed.2011.302. [author reply 1215-1216] [DOI] [PubMed] [Google Scholar]
- 8.Hou L., Ji B.T., Blair A. Body mass index and colon cancer risk in Chinese people: menopause as an effect modifier. Eur J Cancer. 2006;42(1):84–90. doi: 10.1016/j.ejca.2005.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Z., Hankinson S.E., Colditz G.A. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278(17):1407–1411. [PubMed] [Google Scholar]
- 10.Kuper H., Cramer D.W., Titus-Ernstoff L. Risk of ovarian cancer in the United States in relation to anthropometric measures: does the association depend on menopausal status? Cancer Causes Control. 2002;13(5):455–463. doi: 10.1023/a:1015751105039. [DOI] [PubMed] [Google Scholar]
- 11.Lee A.V., Jackson J.G., Gooch J.L. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol. 1999;13(5):787–796. doi: 10.1210/mend.13.5.0274. [DOI] [PubMed] [Google Scholar]
- 12.Leiba A., Kark J.D., Afek A. Adolescent obesity and paternal country of origin predict renal cell carcinoma: a cohort study of 1.1 million 16 to 19-year-old males. J Urol. 2013;189(1):25–29. doi: 10.1016/j.juro.2012.08.184. [DOI] [PubMed] [Google Scholar]
- 13.Lipworth L., Morgans A.K., Edwards T.L. Renal cell cancer histological subtype distribution differs by race and sex. BJU Int. 2016;117(2):260–265. doi: 10.1111/bju.12950. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Tong G., Tong W., Lu L., Qin X. Can body mass index, waist circumference, waist-hip ratio and waist-height ratio predict the presence of multiple metabolic risk factors in Chinese subjects? BMC Public Health. 2011;11(35) doi: 10.1186/1471-2458-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maestro M.L., del Barco V., Sanz-Casla M.T. Loss of heterozygosity on the short arm of chromosome 3 in renal cancer. Oncology. 2000;59(2):126–130. doi: 10.1159/000012149. [DOI] [PubMed] [Google Scholar]
- 16.Molloy C.A., May F.E., Westley B.R. Insulin receptor substrate-1 expression is regulated by estrogen in the MCF-7 human breast cancer cell line. J Biol Chem. 2000;275(17):12565–12571. doi: 10.1074/jbc.275.17.12565. [DOI] [PubMed] [Google Scholar]
- 17.Qu Y., Chen H., Gu W. Age-dependent association between sex and renal cell carcinoma mortality: a population-based analysis. Sci Rep. 2015;5(9160) doi: 10.1038/srep09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riondino S., Roselli M., Palmirotta R. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J Gastroenterol. 2014;20(18):5177–5190. doi: 10.3748/wjg.v20.i18.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez C., Calle E.E., Patel A.V. Effect of body mass on the association between estrogen replacement therapy and mortality among elderly US women. Am J Epidemiol. 2001;153(2):145–152. doi: 10.1093/aje/153.2.145. [DOI] [PubMed] [Google Scholar]
- 20.Siegel R., Ward E., Brawley O., Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 21.Slattery M.L., Ballard-Barbash R., Edwards S., Caan B.J., Potter J.D. Body mass index and colon cancer: an evaluation of the modifying effects of estrogen (United States) Cancer Causes Control. 2003;14(1):75–84. doi: 10.1023/a:1022545017867. [DOI] [PubMed] [Google Scholar]
- 22.Song M.K., Chung J.S., Lee J.J. High Ki-67 expression in involved bone marrow predicts worse clinical outcome in diffuse large B cell lymphoma patients treated with R-CHOP therapy. Int J Hematol. 2015;101(2):140–147. doi: 10.1007/s12185-014-1719-3. [DOI] [PubMed] [Google Scholar]
- 23.Tang H., Liao Y., Xu L. Estrogen and insulin-like growth factor 1 synergistically promote the development of lung adenocarcinoma in mice. Int J Cancer. 2013;133(10):2473–2482. doi: 10.1002/ijc.28262. [DOI] [PubMed] [Google Scholar]
- 24.Terry P., Giovannucci E., Bergkvist L., Holmberg L., Wolk A. Body weight and colorectal cancer risk in a cohort of Swedish women: relation varies by age and cancer site. Br J Cancer. 2001;85(3):346–349. doi: 10.1054/bjoc.2001.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terry P.D., Miller A.B., Rohan T.E. Obesity and colorectal cancer risk in women. Gut. 2002;51(2):191–194. doi: 10.1136/gut.51.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varela I., Tarpey P., Raine K. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469(7331):539–542. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf L.A., Terry P.D., Potter J.D., Bostick R.M. Do factors related to endogenous and exogenous estrogens modify the relationship between obesity and risk of colorectal adenomas in women? Cancer Epidemiol Biomarkers Prev. 2007;16(4):676–683. doi: 10.1158/1055-9965.EPI-06-0883. [DOI] [PubMed] [Google Scholar]
- 28.Yu C.P., Ho J.Y., Huang Y.T. Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-beta activation. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G., Gu C., Zhu Y. ADIPOQ polymorphism rs182052 is associated with clear cell renal cell carcinoma. Cancer Sci. 2015;106(6):687–691. doi: 10.1111/cas.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang G.M., Luo L., Ding X.M. MicroRNA-126 inhibits tumor cell invasion and metastasis by downregulating ROCK1 in renal cell carcinoma. Mol Med Rep. 2016;13(6) doi: 10.3892/mmr.2016.5160. (5029-2036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G.M., Zhu Y., Ye D.W. Metabolic syndrome and renal cell carcinoma. World J Surg Oncol. 2014;12:236. doi: 10.1186/1477-7819-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinicopathological characteristics of 93 patients for whom IHC was performed.
Clinicopathological characteristics of the 364 enrolled female ccRCC patients.
The association of obesity/overweight with ccRCC risk by stratification of estrogen status.
Logistic regression analysis of the association between obesity/overweight and ccRCC risk in overall and pre-menopausal patients.