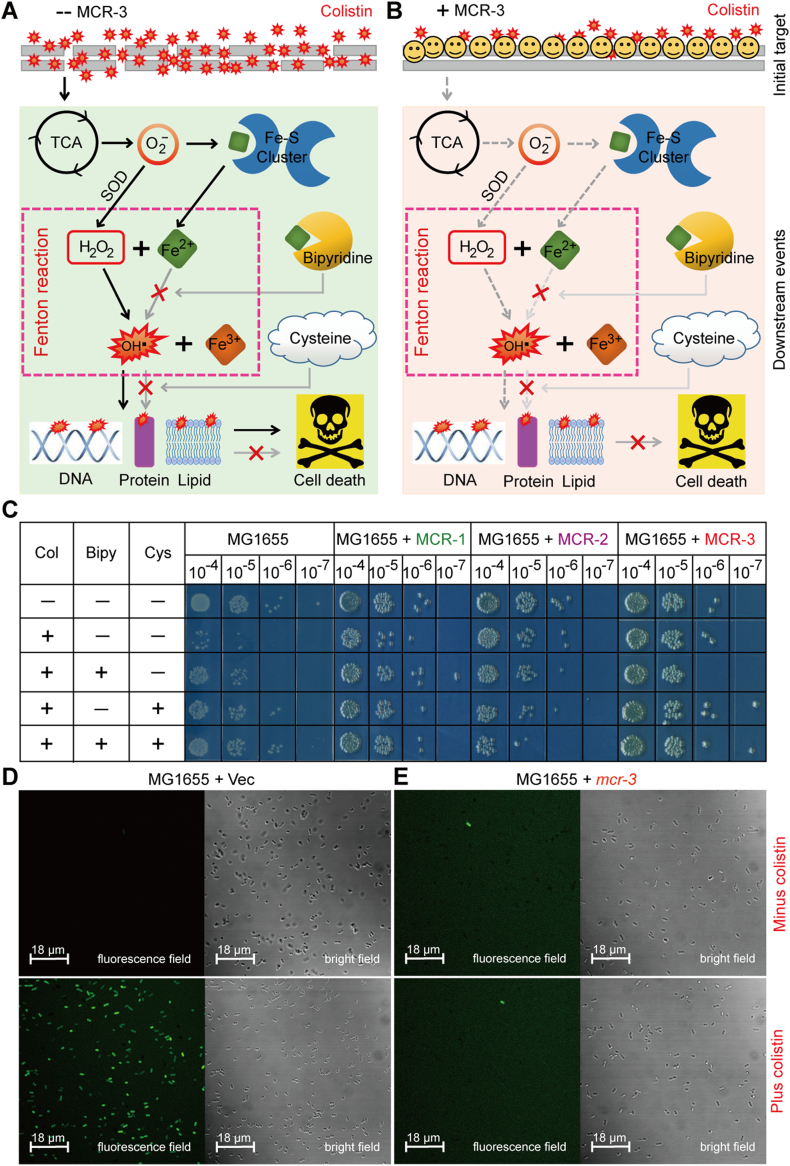
Fig. 8.
A working model for MCR-3-mediated impairment of the hydroxyl radical death pathway in E. coli.
A. Scheme for membrane disruption by the cationic antimicrobial polypeptide colistin and the resultant ROS production in E. coli.
B. Functional expression of MCR-3 prevents the penetration of colistin into bacterial membrane and thereafter attenuates the production of ROS in E. coli.. It was modified appropriately from Wei et al. [44] with permission.
C. Chemical rescue experiments reveal that Fenton reaction involves colistin-triggered hydroxyl radical killing pathway in E. coli.
The LPS-lipid A moiety denotes the first target for colistin treatment. Bipyridine is a well-known ferric chelator, and L-cysteine is the ROS scavenger.
D. Exposure to colistin boosts the accumulation of hydrogen peroxides in the negative control, the strain MG1655 of E. coli with the empty vector pBAD24.
E. The expression of plasmid-borne MCR-3 attenuates the production of hydrogen peroxides in E. coli, regardless of the presence of colistin.
An oxidant-sensitive dye, DCFH2-DA, was used to detect the level of intracellular ROS, which was oxidized by hydrogen peroxides into the fluorescent product of DCF. The fluorescence intensity of DCF was measured with a Zeiss LSM 510 Meta confocal laser scanning microscope (100× oil immersion objective). Hydrogen peroxides appear in green.
F. Quantitative comparison of colistin-promoted ROS levels in E. coli with or without expression of mcr-3.
Ratio of fluorescent cells was calculated through counting the number of cells with/without fluorescence. In each group, no <500 cells were counted from 4 individual photographs. The data was evaluated through one-way analysis of variance (ANOVA) as well as Tukey–Kramer multiple comparisons post hoc test.