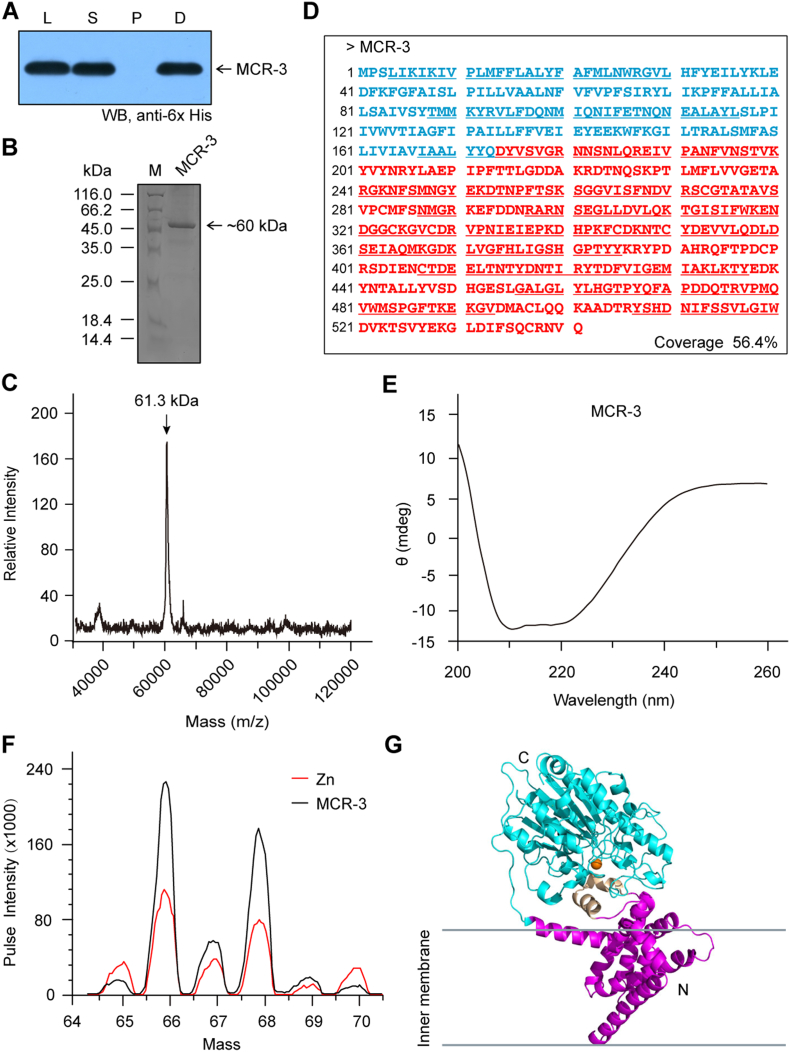
Fig. S6.
Characterization of MCR-3 protein.
A. Western blot detection suggests sub-cellular localization of MCR-3 in the membrane-associated fraction from E. coli lysates Designations: L, Lysate of E. coli following sonication; S, Supernatant of bacterial crude extracts undergoing ultra-centrifugation; P, Pellet of bacterial crude extracts undergoing ultra-centrifugation; D, Detergent-solubilized fraction. B. SDS-PAGE (15%) profile of the purified MCR-3 C. Use of MALDI-TOF to determine the molecular mass of MCR-3 The mass of MCR-3 is unveiled to be 61.3 kDa. D. MS identification of the recombinant MCR-3 integral membrane protein. The transmembrane region of MCR-3 is in light blue, and the enzymatic domain is in red. The polypeptides that have been identified by MS and match MCR-3 are underlined with coverage of 56.4%. E. Circular dichroism (CD)-based illustration of the protein secondary structure of MCR-3. F. Use of ICP/MS to determine the existence of MCR-3 in the zinc-bound form G. Overall architecture of the modelled MCR-3 structure. Abbreviations: C, C-terminus; N, N-terminus.