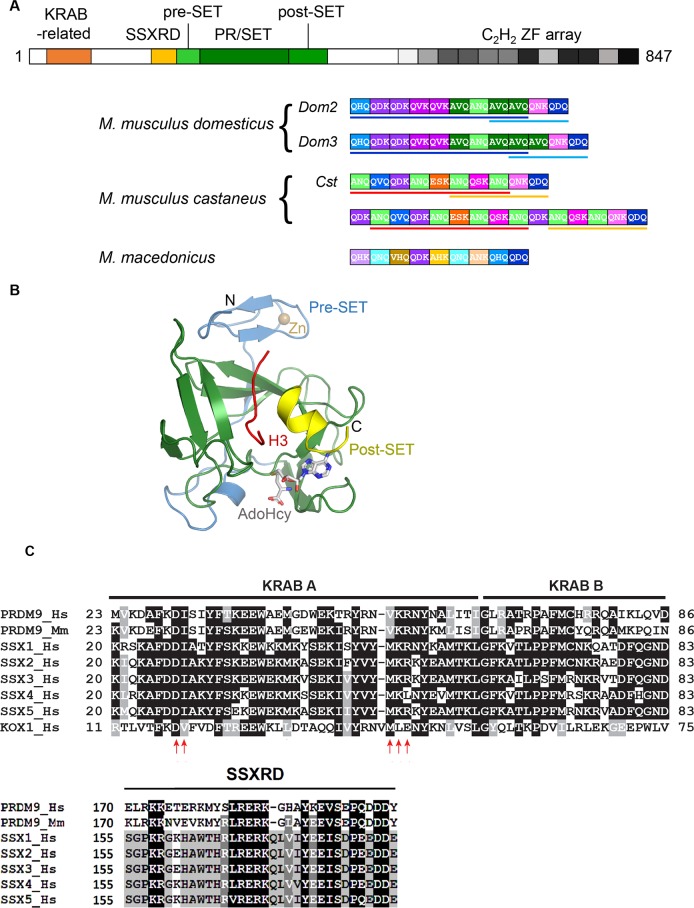
Fig 2. PRDM9 domains.
(A) The domain organization of PRDM9 and the high diversity of its zinc finger array. Top, schematic representation of the mouse PRDM9 protein. Bottom, zinc finger arrays from two Mus musculus domesticus alleles (Dom2 and Dom3 from C57BL/6 and C3H lab strains, respectively), two M. m. castaneus alleles (including Cst from CAST strain) and one representative allele from Mus macedonicus (all alleles described in [31]) are shown underneath. Each box represents one zinc finger, and each color represents a specific zinc finger sequence. The DNA-contacting amino acids in position −1, 3, and 6 of the zinc finger are indicated within each box. Colored bars underline blocks of zinc fingers conserved between alleles from the same subspecies. The recurrence of several individual zinc fingers is apparent within each array. Alleles from the same species (M. musculus) are largely made of combinations of the same set of zinc fingers; conversely, a majority of zinc fingers is not shared between M. musculus and M. macedonicus. (B) Structure of the mouse PRDM9 PR/SET domain (residues 198–368) in complex with a H3K4me2 peptide (H3) and AdoHcy. The SET domain (residues 245–358) is shown in green, the pre-SET domain in blue, and the truncated post-SET in yellow (gift from Jan Kadlec). Pre-SET and post-SET are a zinc knuckle and a zinc finger, respectively, involved in the organization of the SET domain. (C) The KRAB-related and SSXRD domains. PRDM9 shares similarity with the KRAB-related and SSRD domains of the SSX protein family (SSX1 to 5). KOX1 that contains a canonical KRAB domain and interacts with TRIM28 is also shown. Amino acid substitutions that abolish the interaction between KOX1 and TRIM28 are shown by red arrows (reprinted from [49]). The alignment shows similarities in these two domains (white on black: 100% identity, white on grey: 80% identity, black on grey: 60% identity, black on white: less than 60% identity). AdoHcy, Adenosyl-homocysteine; CAST, CAST/Eij mouse strain; H3K4me2, Histone H3 lysine4 dimethyl; Hm, Homo sapiens; KOX1, Krüppel-associated box 1 protein; KRAB, Krüppel-associated box; Mm, Mus musculus; PRDM9, PR domain-containing protein 9; SET, Suppressor of variegation 3–9, Enhancer of Zeste and Trithorax; SSXRD, synovial sarcoma, X breakpoint repression domain; TRIM28 Tripartite motif-containing 28.