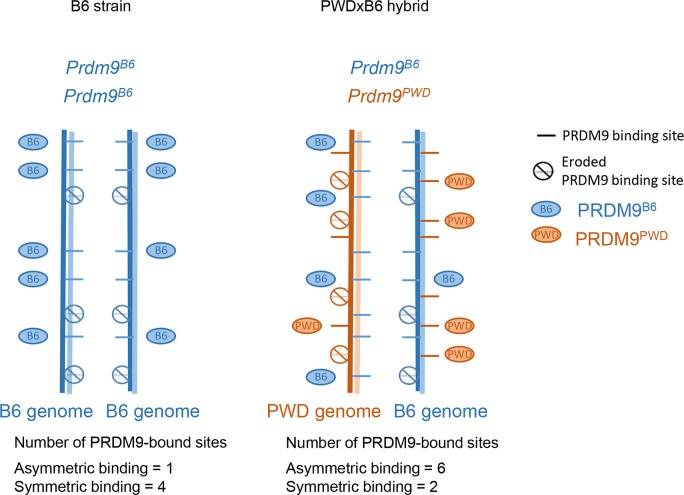
Fig 5. Heterozygosity at Prdm9 and genetic incompatibility.
When Prdm9 is heterozygous (two alleles with different zinc finger domains), DSB sites can be specified by either allele, and the contribution of each allele to DSB formation is not always even but can vary from 50/50 due to the expression level of Prdm9 alleles and/or to the density, affinity, or accessibility of binding sites. Due to hotspot erosion and to a lesser extent to mutations (see Fig 4), in hybrids between strains with divergent genomes, such as Mus musculus domesticus and M. m. musculus, and that have been in contact with specific Prdm9 alleles for generations, some PRDM9 binding sites have been eroded specifically on one genome and are thus heterozygous. An example of a hybrid between the PWD and C57BL/6 (B6) strains (PWDxB6) is shown (right panel). It has been hypothesized that PRDM9 plays a role in DSB repair and that PRDM9 binding on both homologues is required for efficient DSB repair and proper homologous synapsis [73]. According to this assumption, heterozygous sites will differ from homozygous ones by having only one homologue bound with PRDM9. However, if PRDM9 level and/or affinity is low, PRDM9 may bind only on one homologue even at homozygous sites (B6, left panel). Such Prdm9 heterozygous contexts have been described in both humans and mice, but they usually do not seem to influence meiosis and fertility [60, 62, 65, 72, 73, 75]. DSB quantification by DMC1 ChIP-Seq [73] revealed that heterozygous sites (also called asymmetric) have higher levels of DMC1, and this was interpreted as a delay or lower efficiency in DSB repair. The alternative possibility is that more DSBs are induced at such heterozygous sites. In one specific intersubspecific hybrid mouse with a specific genetic background (the PWDxB6 hybrid in which PWD represents M. m. musculus and B6 M. m. domesticus) and in which two different Prdm9 alleles are present, males are sterile. This hybrid sterility context was discovered [144] and analysed in details by J. Forejt’s group [78, 145], who proposed that Prdm9 could be a speciation gene [146]. The proportion of DSB events within asymmetric sites in PWDxB6 hybrids reaches 72%. This is higher than in other hybrids and suggests that a threshold of DSBs made at sites of symmetric PRDM9 binding is not reached in this context, resulting in the synaptic defect and sterility [73, 117]. It was also observed that the proportion of DSBs in “default sites” is increased in these hybrids compared to parental strains [72]. This could provide an alternative interpretation for the DSB repair inefficiency given the properties of these default sites (see Box 1). ChIP-Seq, chromatin immuno-precipitation followed by sequencing; DSB, DNA double strand break; PRDM9, PR domain-containing protein 9.