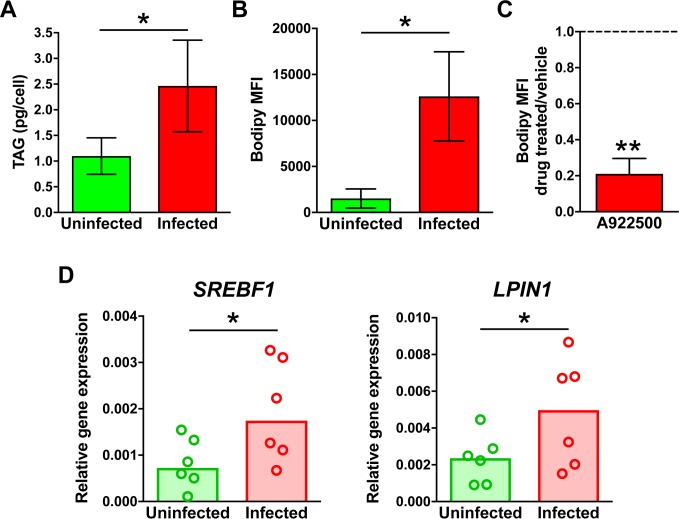
Fig 3. TAG and lipid droplet levels, and expression of TAG metabolism genes in macrophages infected with M. tuberculosis in vitro.
Human monocyte-derived macrophages (MDM) were infected with M. tuberculosis for 24 h or left uninfected. Infected cells were treated with either DMSO (vehicle control) or A922500 (DGAT inhibitor). (A) TAG measurement by mass spectrometry. Lipids were extracted from uninfected and infected cells, and TAG species quantified by LC-MS. (B) Lipid droplet content determination by imaging flow cytometry. Uninfected and infected macrophages were stained with Bodipy 493/503 and imaged by ImageStreamXMark II imaging flow cytometer at 60× magnification. Images were analyzed by IDEAS software and lipid droplet content was expressed as median Bodipy fluorescence intensity per cell (MFI) (the baseline measurements in uninfected cells reflect the scanty lipid droplet induction occurring during macrophage differentiation in vitro). (C) Effect of A922500 on lipid droplet content. Lipid droplet content of infected macrophages was determined as described. Results were expressed as ratio between inhibitor- and vehicle-treated cells. In A, B, and C, average and standard deviation of three donors are shown. (D) Gene expression analysis. RNA was isolated from uninfected and infected cells, and mRNAs enumerated by qPCR using gene-specific primers and molecular beacons (S5 Table). Gene expression was calculated using the 2 -ΔΔCt method and normalized to the housekeeping ACTB gene. Graphs show the medians of six donors, with each dot representing one donor. Statistical significance (*p < 0.05, **p < 0.01) was assessed by paired (panels A, B, and D) and one-sample (panel C) student t-tests. The comparisons in the paired tests are as indicated; the comparison in the one-sample student t-test was between treated and untreated cells. SREBF1 encodes SREBP-1c, a transcription factor that functions as master regulator of TAG biosynthesis [106]; LPIN1 encodes LIPIN1, a TAG biosynthetic enzyme [107] that also functions as transcriptional coactivator of SREBP-1c [108].