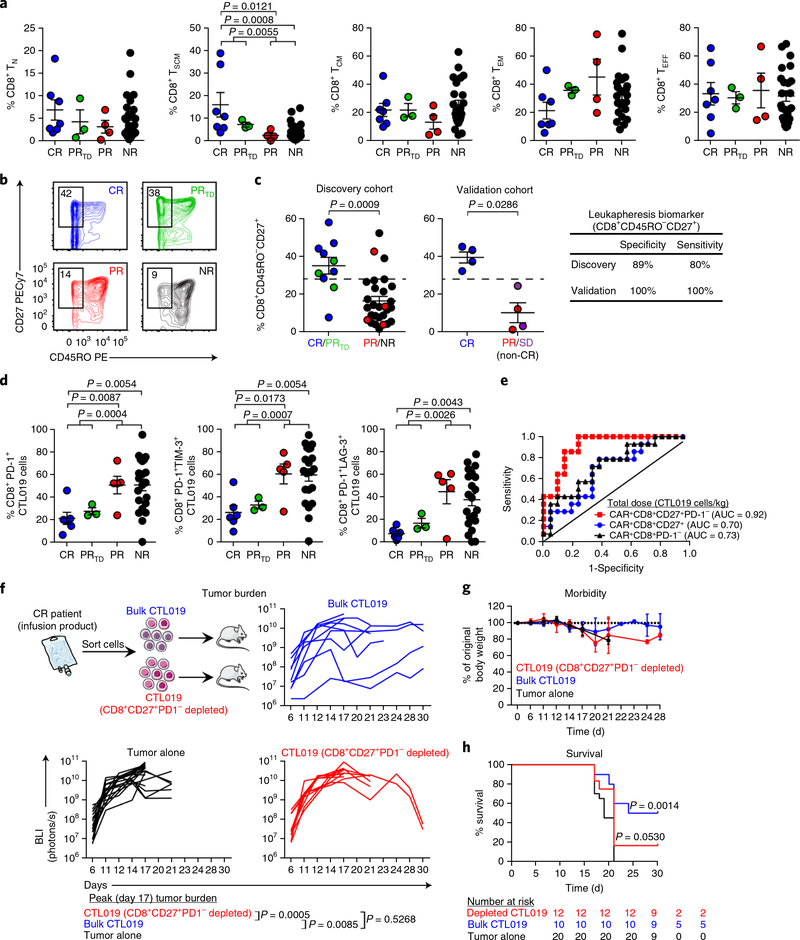
Fig. 4|. The presence of mechanistically relevant T cell populations in patients can predict response to CTL019 therapy.
a, Frequencies of naive (TN), stem cell memory (TSCM), central memory (TCM), effector memory (TEM) and effector (TEFF) CD8+ T cells from patients in each response group (CR, n = 7; PRtd, n = 3; PR, n = 4; NR, n = 24) at the time of leukapheresis. b, Representative contour plots examining the proportions of CD45RO-CD27+CD8+ T cells from CR, PRtd, PR and NR CTL019 patients (3 independent experiments). c, Summary of leukapheresis CD27+CD45RO-CD8+ T cell frequencies in patients belonging to each response category in an original discovery cohort (left). A cutoff value of 28.6% (represented by the dashed lines) optimally segregated patients with highly functional (CR/PRtd; n = 10) or poorly functional (PR/NR; n = 28) CAR T cells (positive predictive value, 72.73%; negative predictive value, 92.59%). A CD45RO-CD27+CD8+ T cell frequency >28.6% segregated CR (n = 4) from non-CR (SD (stable disease) + PR; n = 4) patients with CLL in a validation cohort (middle). Clinical-response assessments for the validation cohort were conducted 3 months after CTL019 cell infusion and are based on tumor-burden status in the lymph nodes and spleen. Specificity and sensitivity measurements for each patient group are displayed in tabular form (right). d, Percentages of CD8+ T cells expressing PD-1 and coexpressing PD-1 and LAG-3 or TIM-3 in preinfusion CTL019 cells (CR, n = 6; PRtd, n = 3; PR, n = 5; NR, n = 21). For a, c and d, two-tailed Mann-Whitney tests were used for comparisons between groups. Graphs show mean and s.e.m. e, Receiver operating characteristic (ROC) curve for multiple biomarkers that define different T cell populations in the CAR T cellular product. ROC curves are based on the total dose (cells/kg) of CTL019 cells infused into CR (n = 6) versus NR patients (n = 21). AUC values, shown in parentheses, represent areas under the respective ROC curves and provide an overall measure of predictive power. f, Top, experimental setup, showing transfer of retrospective CR CAR T cell infusion products with (bulk) or without CD8+CD27+PD-1- lymphocytes (depleted) from patients with CLL into fully leukemic NSG mice (tumor alone, n = 20; bulk CTL019, n = 10; depleted CTL019, n = 12). Bottom, longitudinal tumor burden (BLI) of NALM-6-bearing mice treated with CAR T cells (each line indicates one mouse). g, Weight loss in mice, shown over time. h, Kaplan-Meier curves showing survival of mice. P values were calculated with twotailed log-rank Mantel-Cox tests. Numbers of mice at risk in each group at specific time points are listed below the survival curve.