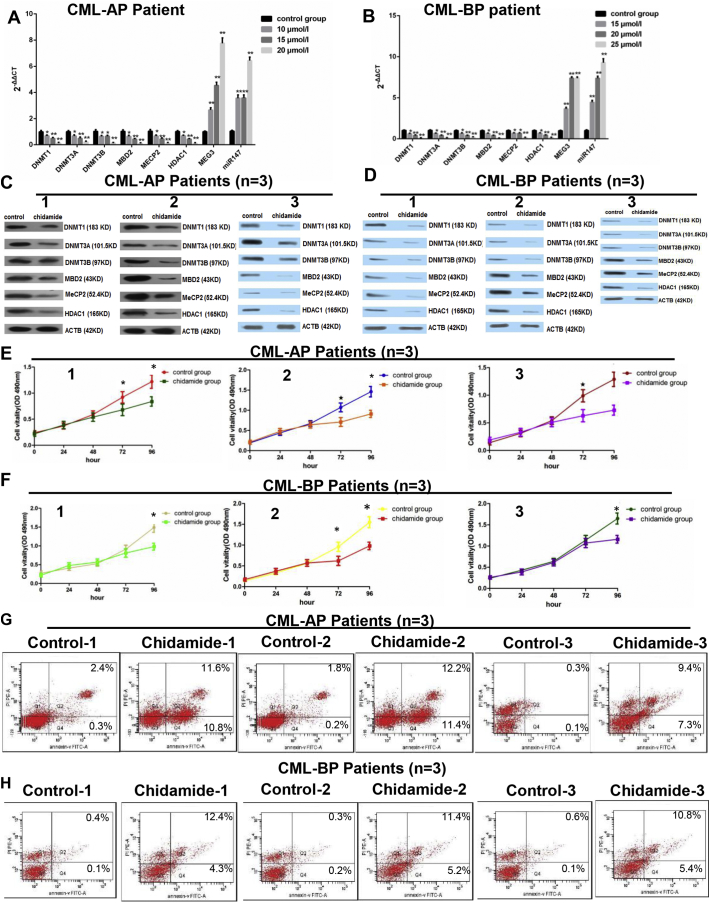
Supplementary Fig. 1.
Chidamide treatment in CML patient-derived cells. A cohort of CML patients was recruited, the bone marrow samples were collected from 3 CML-AP patients and 3 CML-BP patients, and then the peripheral blood mononuclear cells were isolated via a lymphocyte separation. A and B. These patient-derived cells were treated with chidamide at 3 doses (10 μmol/l, 15 μmol/l or 20 μmol/l) and the mRNA expressions of DMNT1, DMNT3A, DMNT3B, MECP2, HDAC1, MEG3 and miR-147 in CML-AP and CML-BP patients were assessed by real-time PCR. C and D. The protein expression levels of these targets were also assessed after chidamide treatment in CML-AP and CML-BP patients using a western blot. E and F. The effect of chidamide treatment on the proliferation of the patient-derived cells was assessed by an MTT assay. G and H. The effect of chidamide treatment on the apoptosis of the patient-derived cells was assessed by an AnnexinV-FITC and PI kit. *: P < 0.05 compared to the control group. **: P < 0.01 compared to the control group. For Supplementary 1A and B, the one-way ANOVA was used for data analysis. For Supplementary 1E and F, Student's t-test was used for data analysis. The comparison of cell apoptosis rate between control group and chidamide group was analyzed using chi-squared test.