Figure 3. The sequential binding of regulatory nucleotides drives SAMHD1 tetramerization and catalytic activation.
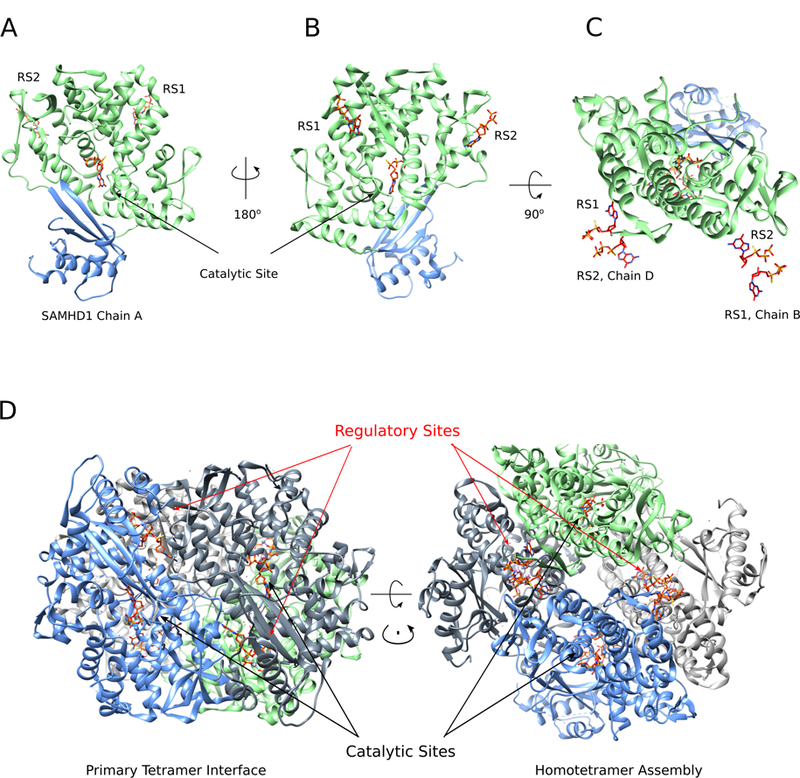
(A, B) SAMHD1 monomer depicting the HD domain major lobe (green) and C-terminal region (blue) (PDBID: 4BZC (71)). The catalytic site and regulatory site 1 (RS1) and 2 (RS2) are indicated with bound dGTP (C) SAMHD1 monomer as in A and B, with paired nucleotides from the tetrameric regulatory cleft. Each cleft contains two regulatory nucleotide binding sites from adjacent monomers that stabilize subunit interactions. (D) The catalytically active tetrameric holoenzyme of SAMHD1 reveals the primary tetramer interface (left) and the homotetrameric assembly (right).
