Abstract
Osteoporosis and cognitive impairment, which are highly prevalent conditions in elderly populations, share several risk factors. This study aims at evaluating the association of bone mineral density (BMD) with prevalent and incident cognitive impairment after a 3-year follow-up. We studied 655 community-dwelling women aged 65+ participating in the InCHIANTI study, who had been followed for 3 years. Total, trabecular, and cortical BMD were estimated by peripheral quantitative computed tomography using standard transverse scans at 4 and 38 % of the tibial length. Cognitive performance was evaluated using the Mini-Mental State Examination and the Trail Making Tests (TMT) A and B; a MMSE score <24 was adopted to define cognitive impairment. The TMT A–B score was calculated as the difference between TMT-A and TMT-B times (ΔTMT). The association of cognitive performance after 3 years with baseline indices of BMD was assessed by logistic and linear regression analyses. Cortical, but not trabecular, BMD was independently associated with incident cognitive impairment (OR 0.93, 95 % CI 0.88–0.98; P = 0.012), worsening cognitive performance (OR 0.96, 95 % CI 0.92–0.98; P = 0.039), and worsening performance in ΔTMT (OR 0.96, 95 % CI 0.92–0.99; P = 0.047). Increasing cortical BMD tertiles was associated with decreasing probability of incident cognitive impairment (P for linear trend =0.001), worsening cognitive performance (P = 0.013), and a worsening performance below the median value (P for linear trend <0.0001). In older women, low BMD might represent an independent and early marker of subsequent cognitive impairment. Physicians should assess and monitor cognitive performance in the routine management of elderly women with osteoporosis.
Keywords: Bone mineral density, Cognitive performance, Elderly, Epidemiology
Introduction
Cognitive dysfunction is a prevalent public health problem. In fact, dementia has been detected in 14–20 % of older subjects; approximately 4.6 million people are diagnosed with dementia each year, with a twofold increase in the disease prevalence every 20 years [1]. By 2020, the population with a diagnosis of dementia is expected to increase to 42.3 million worldwide, and to 81.1 million by 2040 [1]. The incidence of dementia disorders increases exponentially beyond the age of 65. In older populations, cognitive dysfunction has been associated with several adverse outcomes; thus, dementia imposes a significant burden to health care and social costs, and represents a significant fraction of the healthcare costs attributed to older population strata [2].
Due to its worldwide prevalence, osteoporosis is as well a serious public health concern. It has been estimated that over 200 million people worldwide suffer from this condition, approximately one-tenth of women aged 60, one-fifth of women aged 70, two-fifths of women aged 80, and two-thirds of women aged 90 [3].
Low BMD and cognitive impairment share several common risk factors, and previous studies found an association of osteoporosis with stroke, brain atrophy, brain white matter changes, and silent infarcts [4–6]; also, it has been reported that low BMD and cognitive impairment often coexist, and that osteoporosis is associated with increased rates of progression from mild cognitive impairment to Alzheimer’s disease, as well as with incident Alzheimer disease [7, 8]. In addition, there is evidence of an increased risk of dementia in subjects with diagnosis of osteoporosis or osteoporotic fractures in Asian populations [1]; however, there are relevant racial differences in bone mass, so that Asian women have lower bone mass than Caucasian [9].
The aim of the present study was to evaluate the association of BMD with prevalent and incident cognitive impairment over a 3-year follow-up in a population of community-dwelling elderly living in two small towns in the countryside of Tuscany, Italy, who had been enrolled in the Invecchiare in CHIANTI study.
Methods
Study Design and Participants
The present study is based upon data from the “Invecchiare in Chianti” study, a prospective population-based study of older persons in Tuscany, Italy that aims to identify risk factors for late-life disability [10]. Baseline data collection started in September 1998, and was completed in March 2000. The Italian National Research Council on Aging Ethical Committee ratified the study protocol, and participants provided written consent to participate.
At baseline, analyses for the present study included all 655 women aged 65+. After three years of follow-up, data were available for 353 women without baseline cognitive impairment (we excluded from the original sample 249 participants with baseline diagnosis of dementia (cortical or vascular)), with a baseline MMSE score <24 or with baseline incomplete data on bone parameters or cognitive function, and 53 women because they were lost at the 3 year follow-up evaluation, or had incomplete data for this study.
Cognitive Function
Cognitive performance was assessed adopting the 30-item MMSE, which is a most widely used tool for measuring of cognitive function; it has been proven as an effective screening instrument for cognitive impairment in general populations [11]. Scores for the MMSE range from 0 to 30, with lower scores reflecting worse cognitive function. A cutoff of 24 was used to detect cognitive impairment; in addition, worsening cognitive performance was considered for subjects with lower scores at the follow-up tests, as compared with baseline evaluations (i.e., MMSE at follow-up — MMSE at baseline = ΔMMSE < 0); furthermore, to avoid any casual fluctuation in cognitive performance, among subjects with a worsening cognitive performance, a performance below the median value was also considered. All the MMSE scores were adjusted according to the following formula [(unadjusted MMSE/maximum possible point for completed items) × 30] [12].
Also, the Trail Making Test (TMT) was administered to measure visuospatial scanning, sequential processing, motor speed, attention, and executive functioning. The Trail Making Test is administered in two parts [13]. Part A is a visual-scanning, a timed task in which participants are asked to connect with lines 25 circles numbered from 1 to 25 as quickly as possible. In Part B participants are asked to connect circles containing numbers or letters in an alternate numeric/alphabetical order. The TMT A-B score calculated as the difference between TMT-A and TMT-B times (ΔTMT) is considered a measure of cognitive flexibility relatively independent of manual dexterity [14, 15]. Worsening performance in ΔTMT was considered for an increased time to carry out the follow-up test, as compared with baseline evaluation (i.e., ΔTMT at followup — ΔTMT at baseline = ΔTMT < 0). Furthermore, to avoid casual fluctuations in cognitive performance among subjects with worsening ΔTMT performance, a performance below the median value was also considered.
Bone Parameters
BMD was estimated using peripheral quantitative computed tomography (pQCT) using an XCT 2000 device (Stratec Medizintechnik, Pforzheim, Germany) at the tibial site. The precision error of the XCT2000 was below 1 % for volumetric trabecular and cortical density. The pQCT was adopted because this technique has been shown to be effective in the assessment of osteoporosis and in predicting the risk of osteoporotic fractures. The tibiotalar joint was identified using a pQCT longitudinal scout and used as an anatomic marker for the identification of measurements sites.
Standard (2.5-mm thickness) transverse scans were obtained at 4 and 38 % of tibial length to measure trabecular and cortical bone density, respectively. The cross-sectional images obtained from the pQCT were analyzed using the BonAlyse software (BonAlyse Oy, Jyvaskyla, Finland), a software for processing pQCT scans that automatically identifies cortical and trabecular bone tissue assessing at the same time the BMD. Areas with density values >710 mg/cm3 were considered as cortical bone, whereas those having density values between 180 and 710 mg/cm3 were considered as trabecular bone [16]. The precision error of the XCT-2000 is below 1 % for volumetric trabecular and cortical density and for cortical bone area [16]. The pQTC bone measures included in the present study were as follows: total bone density (4 and 38 % of tibial length), trabecular bone density (4 % of tibial length), cortical bone density (38 % of tibial length).
Covariates
Smoking was self-reported, and expressed as pack-years. Education was expressed as years of school attendance. Data on dietary intake, including alcohol consumption, were collected by the questionnaire created for the European Prospective Investigation into Cancer and nutrition (EPIC) study [17]. Adherence to the Mediterranean diet was ascertained using the Mediterranean Diet Score [18], as well as the dosage of urinary total polyphenols, which are phytochemical compounds present in plant-based foods, such as fruits, vegetables, and derived foods.
Adjudicated disease diagnoses were based on self-reported history, clinical documentation, and medication use, as well as standardized criteria derived from the Women’s Health and Aging Study protocol [19]. Comorbidity was quantified using the Charlson comorbidity index score [20]. All drugs assumed by participants were coded according to the Anatomical, Therapeutic, and Chemical codes [21]. Depressive symptoms were assessed using the original 20-item version of the Center for Epidemiological Studies-Depression Scale (CES-D) [22]. Blood samples were obtained from participants after 12-h fasting, and after resting for at least 15 min. Aliquots of serum were stored at −80 °C and were not thawed until analyzed. Glomerular Filtration Rate was estimated using the Cock-croft-Gault equation. Functional ability was estimated by self-report using the Katz’ questionnaire investigating Activities of Daily Living [23]. This tool is most commonly adopted for assessing functional independency for clinical and epidemiological purposes. Walking speed was defined as the best performance (m/s) of two 4-m walks at usual pace along a corridor. Participants were allowed to use walkers. Usual physical activity was self-reported and defined as walking inside the house for at least 3 h/day.
Statistical Analyses
Data were recorded using dedicated software. Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS for Mac version 20.0, 2011, SPSS Inc., Chicago, IL); differences were considered significant at the P < 0.050 level.
Data of continuous variables are presented as mean values ± standard deviation (SD). Analysis of variance for normally distributed variables according to incident cognitive impairment (defined as a MMSE < 24) was performed by ANOVA comparisons; medians and interquartile ranges were provided for non-normally distributed variables, and the non-parametric Mann-Whitney U test was adopted. The two-tailed Fisher exact test was used for dichotomous variables.
Logistic regression was used to evaluate the association of prevalent cognitive impairment at baseline with bone mineral density parameters and with increasing BMD tertiles.
In addition, in women without cognitive impairment at baseline, multivariable linear regression analysis was adopted to estimate the crude, as well as the adjusted association of MMSE score at follow-up with baseline BMD parameters; multivariable logistic regression analysis was adopted to estimate the crude, as well as the adjusted association of 3-year incident cognitive impairment with baseline BMD parameters; also, multivariable logistic regression analysis was adopted to estimate the crude, as well as the adjusted association of the prevalence of worsening cognitive performance with baseline BMD parameters; the covariates which were entered in the multivariable models were those which differed significantly (P < 0.050) in univariate analyses.
Also, logistic regression analysis was adopted to evaluate the association of increasing cortical and total BMD tertiles with incident cognitive impairment after the 3 years follow-up. Moreover, analysis of the interaction term “cortical BMD × age” was also performed to assess whether the association of cortical BMD with incident cognitive impairment, prevalent worsening cognitive impairment, as well as prevalent worsening performance in ΔTMT varied according to age.
Eventually, logistic regression was adopted to assess the adjusted association of prevalent worsening performance in ΔTMT with baseline bone parameters.
Results
Baseline cortical BMD tertiles were <1065 mg/cm3 (lower), 1065–1128 mg/cm3 (medium), and >1128 mg/cm3 (higher). The corresponding intervals for total BMD at 38 % of the tibial length were <820 mg/cm3, 820–903 mg/cm3, and >903 mg/cm3.
Cross-Sectional Analysis
Baseline cognitive impairment was detected in 249/655 (38 %) women. The mean ΔTMT was 106 ± 68 s. The mean age of women with cognitive impairment was significantly higher as compared with others (80 ± 8 vs. 73 ± 6; P < 0.0001).
Cortical BMD and total BMD obtained at 38 % of the tibial length were significantly lower in women with cognitive impairment (cortical BMD at 38 % of the tibial length: 1067 ± 89 vs. 1088 ± 75 mg/cm3; P = 0.006; total BMD at 38 % of the tibial length: 828 ± 106 vs. 862 ± 91 mg/cm3; P < 0.0001), while there were no differences in trabecular and total BMD at 4 % of the tibial length (trabecular BMD at 4 % of the tibial length: 190 ± 69 vs. 197 ± 54 mg/cm3; P = 0.179; total BMD at 4 % of the tibial length: 231 ± 56 vs. 239 ± 43 mg/cm3; P < 0.074). Also, cortical BMD was lower in women with a mean ΔTMT below the mean value (1083 ± 75 vs. 1112 ± 68 mg/cm3; P = 0.030), while no association was found for other BMD parameters (total BMD at 4 % of the tibial length: 868 ± 92 vs. 885 ± 82 mg/cm3; P = 0.282; total BMD at 4 %: 241 ± 42 vs. 240 ± 35 mg/cm3; P = 0.781; trabecular BMD: 199 ± 60 vs. 202 ± 49 mg/cm3; P < 0.731).
Longitudinal Analysis
The main characteristics of participants according to incident cognitive impairment after 3 years are depicted in Table 1.
Table 1.
Characteristics of participants aged 65+ according to the development of cognitive impairment
| Participants with incident cognitive impairment (n = 76) |
Participants without incident cognitive impairment (n = 277) |
P | |
|---|---|---|---|
| Demographics and lifestyle habits | |||
| Age (years) | 77 ± 7 | 72 ± 6 | <0.0001 |
| Education (years) | 4 ± 2 | 6 ± 3 | <0.0001 |
| Smokinga | 0 (0–0) | 0 (0–6) | 0.001 |
| Self-perceived low income | 35 (47 %) | 163 (59 %) | 0.066 |
| Living alone | 42 (55 %) | 121 (44 %) | 0.091 |
| Falls in the previous year | 28 (37 %) | 68 (24 %) | 0.041 |
| Usual physical activityb | 58 (76 %) | 227 (82 %) | 0.324 |
| Protein intake (g/day) | 66 ± 17 | 72 ± 20 | 0.019 |
| Calcium intake (g/day) | 759 ± 233 | 833 ± 352 | 0.087 |
| Energy intake (Kcal/day) | 1659 ± 419 | 1780 ± 513 | 0.060 |
| Alcohol consumption (g/day) | 1.8 (0–13.3) | 1.9 (0–13.2) | 0.665 |
| Mediterranean Diet Score | 4 ± 2 | 5 ± 2 | 0.238 |
| Comorbid conditions | |||
| Diabetes | 12 (16 %) | 18 (6 %) | 0.018 |
| Hypertension | 48 (63 %) | 171 (62 %) | 0.894 |
| Atrial fibrillation | 4 (6 %) | 4 (1 %) | 0.063 |
| Heart failure | 4 (5 %) | 8 (3 %) | 0.297 |
| Chronic pulmonary disease | 0 | 3 (1 %) | 0.999 |
| Stroke | 4 (5 %) | 11 (4 %) | 0.539 |
| Parkinson’s disease | 3 (4 %) | 2 (1 %) | 0.069 |
| Hepatic disease | 1 (1 %) | 1 (1 %) | 0.385 |
| Visual impairment | 5 (9 %) | 7 (3 %) | 0.051 |
| Hearing impairment | 5 (7 %) | 7 (2 %) | 0.141 |
| Charlson comorbidity score index | 1 (0–1) | 1 (0 ± 1) | 0.225 |
| Medications | |||
| Neuroleptics | 6 (8 %) | 6 (2 %) | 0.025 |
| SSRIc | 2 (3 %) | 3 (1 %) | 0.294 |
| ACE-inhibitors | 16 (21 %) | 31 (11 %) | 0.035 |
| Antiplatelets | 11 (14 %) | 17 (6 %) | 0.028 |
| Anticoagulants | 0 | 5 (2 %) | 0.589 |
| L-thyroxine | 1 (1 %) | 16 6(%) | 0.136 |
| Benzodiazepines | 14 (18 %) | 63 (23 %) | 0.531 |
| Loop diuretics | 7 (9 %) | 20 (7 %) | 0.626 |
| Corticosteroids | 2 (3 %) | 4 (1 %) | 0.613 |
| Bisphosphonates | 3 (4 %) | 9 (3 %) | 0.726 |
| Biohumoral parameters | |||
| Glomerular Filtration Rate | 58 ± 19 | 66 ± 17 | 0.002 |
| Total proteins (g/dL) | 7.2 ± 0.4 | 7.1 ± 0.4 | 0.728 |
| Interleukin 6 (pg/mL) | 1.3 (0.7–2.1) | 1.9 (0.7–1.9) | 0.101 |
| CRP-HS (μg/mL)d | 2.3 (1.5–4.5) | 2.5 (1.3–5.1) | 0.294 |
| Hemoglobin (g/dl) | 13.3 ± 0.9 | 13.3 ± 1.0 | 0.634 |
| Total cholesterol (mg/dL) | 220 ± 36 | 232 ± 34 | 0.017 |
| 25(OH) vitamin D | 32.8 (21.1–43.0) | 38.7 (26.3–55.7) | <0.0001 |
| Parathyroid hormone (pg/mL) | 26.2 (17.9–43.0) | 23.2 (16.0–55.7) | 0.008 |
| Activities of Daily Living | 5 ± 1 | 6 ± 0 | 0.100 |
| CES-De | 17 ± 9 | 14 ± 9 | 0.005 |
| DHEAS (μg/dL)f | 61.2 (36.1–105.1) | 63.6 (40.4–116.0) | 0.237 |
| 17 beta-estradiol (pg/mL) | 5.7 (4.3–8.7) | 5.5 (3.9–7.7) | 0.604 |
| Urinary Polyphenolg | 197.9 ± 67.6 | 194.5 ± 80.0 | 0.771 |
| Physical and cognitive performance | |||
| Baseline Mini–Mental State Examination | 25 ± 2 | 27 ± 2 | <0.0001 |
| Gait speed (m/sec) | 0.9 ± 0.2 | 1.0 ± 0.2 | <0.0001 |
| Bone pQTC parameters | |||
| Trabecular bone mineral density (mg/cm3) | 200 ± 56 | 197 ± 53 | 0.754 |
| Cortical bone mineral density (mg/cm3) | 1052 ± 79 | 1096 ± 73 | <0.0001 |
| Total bone mineral density at 38 % of the tibial length (mg/cm3) | 824 ± 88 | 871 ± 90 | <0.0001 |
| Total bone mineral density at 4 % of the tibial length (mg/cm3) | 237 ± 43 | 239 ± 43 | 0.767 |
Pack-years
Walking inside the house for at least 3 h/day
Selective Serotonin Reuptake Inhibitor
High-sensitivity C-reactive protein
20-item version of the Center for Epidemiological Studies-Depression Scale
Dehydroepiandrosterone sulfate
Expressed as a mg of gallic acid eq/g creatinine
Incident cognitive impairment was found in 76/353 (21 %) participants. The baseline MMSE score was significantly lower in subjects who developed cognitive impairment as compared with other participants (25 ± 2 vs. 27 ± 2; P < 0.0001); after the 3-year follow-up, the mean MMSE was 20 ± 3 among participants with cognitive decline, and 27 ± 2 (P < 0.0001) in other subjects. Also, 179 (44 %) of women had a worsening performance at the follow-up MMSE as compared with the baseline testing; among these participants the median variation in the MMSE score was −2 (−4; −1).
The mean ΔTMT at follow-up was 82 s, and 58 (17 %) of women exhibited a worse ΔTMT, as compared with the baseline evaluation. Among participants with a worse ΔTMT, the median variation was 32 (12–57) s.
At baseline, both the cortical and total BMD obtained at 38 % of the tibial length were significantly lower in subjects who developed incident cognitive impairment (cortical BMD at 38 % of the tibial length: 1052 ± 79 vs. 1096 ± 73 mg/cm3; P < 0.0001; total BMD at 38 % of the tibial length: 824 ± 88 vs. 871 ± 90 mg/cm3; P < 0.0001). No significant association was found for trabecular and total BMD at 4 % of the tibial length.
Baseline cortical BMD at 38 % of the tibial length was the only BMD parameter significantly associated with a follow-up ΔTMT below the mean value (1078 ± 76 vs. 1114 ± 69 mg/cm3; P = 0.010), while no association was found for total BMD obtained at 38 % of the tibial length (861 ± 92 vs. 887 ± 81 mg/cm3; P = 0.108), nor for trabecular (197 ± 62 vs. 204 ± 49 mg/cm3; P = 0.468) or total BMD at 4 % of the tibial length (240 ± 44 vs. 240 ± 45 mg/cm3; P = 0.892).
Multivariable Analysis
Analyses of crude and multivariable linear regression models indicated that the baseline total BMD at 38 % of the tibial length was associated with the follow-up MMSE score in women without baseline cognitive impairment (crude model: B = 0.10, 95 % CI 0.05–0.14; P < 0.0001. Multivariable model: B = 0.05,95 % CI 0.01–0.09; P = 0.019). Likewise, cortical BMD was associated with the follow-up MMSE score in the crude (B = 0.01, 95 % CI 0.07–0.18; P < 0.0001), as well as in the multivariable model (B = 0.08, 95 % CI 0.03–0.13; P = 0.001). No significant association was found between the follow-up MMSE score and total BMD at 4 % of the tibial length (crude model: B = 0.07, 95 % CI −0.01 to 0.02; P = 0.133) or the trabecular BMD (crude model B = 0.03, 95 % CI −0.04 to 0.11; P = 0.410) (Table 2).
Table 2.
Association (B coefficients, B, or Odds Ratios, OR, and 95 % confidence intervals, CI) of cognitive performance after the 3-year follow-up with the variables of interest according to linear and logistic regression analyses
| Bone Mineral Density (mg/cm3) | Cognitive performance after the 3-year follow-up | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mini-Mental State Examination score |
Incident cognitive impairmenta |
Worsening cognitive performanceb |
|||||||
| B | 95 % CI | P | OR | 95 % CI | P | OR | 95 % CI | P | |
| Total 38 % tibial | |||||||||
| Crude association | 0.10 | 0.05-0.14 | <0.0001 | 0.95 | 0.92-0.98 | <0.0001 | 0.97 | 0.94-0.98 | 0.012 |
| Fully adjustedc | 0.05 | 0.01-0.09 | 0.019 | 0.96 | 0.92-1.01 | 0.97 | 0.98 | 0.95-1.01 | 0.201 |
| Cortical | |||||||||
| Crude association | 0.01 | 0.07-0.18 | <0.0001 | 0.93 | 0.90-0.96 | <0.0001 | 0.96 | 0.93-0.98 | 0.008 |
| Fully adjustedc | 0.08 | 0.03-0.13 | 0.001 | 0.93 | 0.88-0.98 | 0.012 | 0.96 | 0.92-0.98 | 0.039 |
| Total 4 % tibial | |||||||||
| Crude association | 0.07 | -0.01-0.02 | 0.133 | 0.99 | 0.93-1.05 | 0.766 | 0.97 | 0.92-1.02 | 0.235 |
| Fully adjustedc | |||||||||
| Trabecular | |||||||||
| Crude association | 0.03 | -0.04-0.11 | 0.410 | 1.00 | 0.96-1.06 | 0.753 | 0.97 | 0.93-1.01 | 0.195 |
| Fully adjustedc | |||||||||
All the covariates were entered simultaneously into the adjusted regression models
Defined as a MMSE score <24
Defined as the prevalence of subjects with a lower MMSE score at the follow-up test, as compared with baseline evaluation (MMSE at followup – MMSE at baseline = ΔMMSE < 0)
Adjusted for: age; education, smoking; falls, protein intake; diagnosis of diabetes; use of neuroleptics, ACE-inhibitors, and antiplatelets; glomerular filtration rate, total cholesterol, hemoglobin, 25(OH) vitamin D, parathyroid hormone, 20-item version of the Center for Epidemiological Studies-Depression Scale, gait speed, and the baseline Mini–Mental State Examination score
Also, analyses of the crude and multivariable logistic regression models indicated that cortical BMD was inversely associated with the probability of incident cognitive impairment at 3 years (crude model: OR 0.93, 95 % CI 0.90–0.96; P < 0.0001. Multivariable model: OR 0.93, 95 %CI 0.88–0.98; P = 0.012). Also, total BMD at 38 %of the tibial length was inversely associated with incident cognitive impairment only in the crude model (OR 0.95, 95 % CI 0.92–0.98; P < 0.0001). Again, no significant association was found between incident cognitive impairment and total BMD assessed at 4 % of the tibial length (crude model: OR 0.99, 95 % CI 0.93–1.05; P = 0.766); or trabecular BMD (crude model: OR 1.00, 95 % CI 0.96–1.06; P = 0.753). According to multivariable logistic model, age (OR 1.15, 95 % CI 1.03–1.29; P = 0.014), diabetes (OR 7.83, 95 % CI 1.22–10.22; P = 0.030), and baseline MMSE (OR 0.59, 95 % CI 0.43–81; P = 0.001) were also significantly associated with incident cognitive impairment.
Furthermore, analysis of the interaction term indicated that age did not affect the association between cortical BMD and incident cognitive impairment (P = 0.178).
In addition, according to logistic regression, cortical BMD was inversely associated with worsening MMSE scores (crude model: OR 0.96, 95 % CI 0.93–0.98; P = 0.008. Multivariable model: OR 0.96, 95 % CI 0.92–0.98; P = 0.039). The total BMD at 38 % of the tibial length was inversely associated with incident cognitive impairment only in the crude model (OR 0.97, 95 % CI 0.94–0.98; P = 0.012). No significant association was found between prevalence of worsening cognitive performance and total BMD at 4 % of the tibial length (OR 0.97, 95 % CI 0.92–1.02; P = 0.235) or trabecular BMD (OR 0.97, 95 % CI 0.93–1.01; P = 0.195). According to the multivariable logistic model, age (OR 1.08, 95 % CI 1.01–1.15; P = 0.024), education level (OR 0.87, 95 % CI 0.78–0.97; P = 0.013), total cholesterol level (OR 0.91, 95 % CI 0.83–0.99; P = 0.045), as well as baseline MMSE (OR 1.31, 95 % CI 1.09–1.57; P = 0.003) were all associated with prevalent worsening cognitive performance. Moreover, analysis of the interaction term indicated that age did not affect the association between cortical BMD and prevalent worsening cognitive performance (P = 0.491). Again, among subjects with worsening ΔMMSE, worsening below the median value was inversely associated with cortical BMD (crude model: (OR 0.93, 95 % CI 0.90–0.97; P = <0.0001); multivariable model: OR 0.93, 95 % CI 0.88–0.98; P = 0.011); among these subjects, age (OR 1.09, 95 % CI 1.01–1.17; P = 0.020), education level (OR 0.80, 95 % CI 0.65–0.99; P = 0.043), diagnosis of diabetes (OR 7.24, 95 % CI 1.68–12.77; P = 0.008), and baseline MMSE score (OR 1.076, 95 % CI 1.01–1.12; P = 0.018) were all associated with a ΔMMSE score below the median value.
Also, total BMD at 38 % of the tibial length was inversely associated with a worsening cognitive performance below the median value in the crude model (OR 0.95, 95 % CI 0.92–0.98; P = <0.0001) as well as in the multivariable model (OR 0.95, 95 % CI 0.90–0.99; P = 0.026); among these subjects, age (OR 1.08, 95 % CI 1.01–1.17; P = 0.029), education level (OR 0.79, 95 % CI 0.64–0.98; P = 0.036), diagnosis of diabetes (OR 6.49, 95 % CI 1.51–7.84; P = 0.012), and baseline MMSE score (OR 1.06, 95 % CI 1.01–1.12; P = 0.023) were all associated with a ΔMMSE score below the median value.
No significant association was found between worsening cognitive performance below the median value and total BMD at 4 % of the tibial length in the crude model (OR 0.99, 95 % CI 0.93–1.05; P = 0.776) or trabecular BMD (OR 1.01, 95 % CI 0.96–1.06; P = 0.644).
Also, cortical BMD was associated with a reduced probability of worsening performance in ΔTMT in the multivariable model (OR 0.96, 95 % CI 0.92–0.99; P = 0.047), while no significant association was found for total BMD at 38 % of the tibial length (OR 0.98, 95 % CI 0.94–0.1.01; P = 0.185), nor for trabecular BMD (OR 0.98, 95 % CI 0.93–1.03; P = 0.414) or total BMD at 4 % of the tibial length (OR 0.96, 95 % CI 0.90–1.02; P = 0.149). According to the multivariable logistic regression model, education level (OR 0.84, 95 % CI 0.72–0.97; P = 0.019) and baseline ΔTMT (OR 0.97, 95 % CI 0.95–0.98; P = 0.001) were all associated with prevalent worsening performance in ΔTMT. Furthermore, analysis of the interaction term indicated that age did not affect the association between cortical BMD and prevalent worsening performance in ΔTMT (P = 0.092). Among subjects who performed a worse ΔTMT, a performance below the median value was again associated with cortical BMD (OR 0.91, 95 % CI 0.83–0.99; P = 0.033). Education level (OR 0.60, 95 % CI 0.33–0.93; P = 0.025) and baseline ΔTMT (OR 0.97, 95 % CI 0.94–0.99; P = 0.013) were also associated with a performance below the mean value. Conversely, no association was found for total BMD at 38 % of the tibial length (OR 0.96, 95 % CI 0.89–0.1.02; P = 0.170), nor for trabecular BMD (OR 0.91, 95 % CI 0.80–1.02; P = 0.122) or total BMD at 4 % of the tibial length (OR 0.94, 95 % CI 0.79–1.09; P = 0.406).
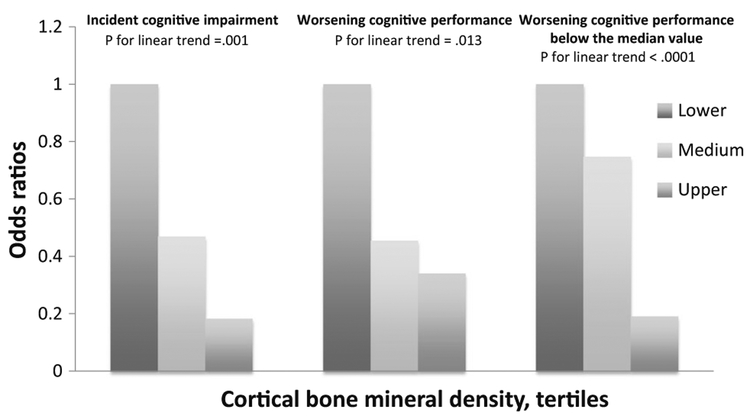
At baseline, increasing cortical BMD tertiles were associated with lowering probability of prevalent dementia (P for linear trend < 0.0001); however, such an association was no longer significant after introducing age (P for linear trend = 0.081); likewise, increasing baseline cortical BMD tertiles were associated with decreasing probability of incident cognitive impairment (P for linear trend = 0.001), worsening cognitive performance (P for linear trend = 0.013), as well a ΔMMSE score below the median value in subjects who exhibited a worse cognitive performance (P for linear trend < 0.0001) (Fig. 1).
Fig. 1.
Association (odds ratios) according to logistic regression analysis in female participants, of increasing cortical BMD tertiles with incident cognitive impairment, worsening cognitive performance, and a performance below the median value in subjects with worsening cognitive performance, after 3 years
Discussion
Results of the present study indicate that bone density, particularly at the cortical level, is independently and inversely associated with dementia and incident cognitive impairment in community-dwelling elderly women. This finding adds to the available evidence on the involvement of common subsystems in cognitive and physical aging, which contribute to the development of the frailty phenotype.
Noticeably, the association of BMD with dementia and incident cognitive impairment suggests that the determinants of decreased bone density at baseline were still acting on cognitive function after 3 years.
Several factors may contribute to explain this association. Hypoxemia has been associated with both dementia and osteoporosis; in fact, low hemoglobin levels and depressed ejection fraction has been linked with low BMD, as well as with cognitive decline [24–27]. Furthermore, hormonal changes, which are involved in the pathophysiology of osteoporosis, have also been associated with the development of dementia. In this setting, estrogen deficiency is of particular interest. In fact, several cross-sectional studies reported larger hippocampus size in women receiving hormone therapy [28]. In addition, use of the estrogen receptor modulator raloxifene, which is administrated for the treatment of osteoporosis, has been associated with improved cognition [29]. Also, studies on human neural cells have demonstrated neurotrophic and neuroprotective effects of dehydroepiandrosterone and its metabolites, mainly through dehydroepiandrosterone-dependent neural stem cell stimulation, genomic activity modulation, and upregulation of androgen receptor levels [29]. In clinical studies, higher dehydroepiandrosterone levels have been related to better executive functioning, and to higher MMSE scores, even though these results are still controversial [30, 31]. However, in the present study dehydroepiandrosterone sulfate and estradiol levels were not significantly associated with incident cognitive impairment. Of notice, our population was older than those enrolled in previous studies. Moreover, while some association between hormonal profile and cognition has been evidenced in cross-sectional studies, longitudinal studies have so far yielded contrasting results [30]; studies on DHEAS supplementation have produced as well inconsistent results [30].
Also, an association has been reported in female subjects between low BMD and the presence of carotid plaques and peripheral artery disease, which are both risk factors for the development of cognitive decline [32].
It has been hypothesized that increased serum levels of proinflammatory cytokines may have a role in the development of both osteoporosis and dementia [33]; nevertheless, in the present study hsCRP and Il-6 levels were not significantly associated with incident cognitive impairment. In addition, low vitamin D levels, which are associated with lower BMD, may increase the risk of dementia through both neurodegenerative and vascular mechanisms [33]; in fact, vitamin D receptors are expressed in areas involved in memory; the active form of vitamin D, 1,25 dihydroxy-vitamin D3, is known to regulate neurotrophin expression, as well as the survival, development, and function of neural cells. In vitro, vitamin D also stimulates macrophages, which increases the clearance of amyloid plaques [34].
From a genetic point of view, an association of the Apo E4 allele with low BMD has been repeatedly reported, yet results are conflicting [1, 35]. In the present study, serum vitamin D levels were lower among participants who developed cognitive impairment; nevertheless, the association of low BMD with cognitive impairment was independent of vitamin levels.
Recently, it has been observed that gait slowing occurs early before the onset of dementia, thus configuring the “motoric cognitive risk syndrome” [36]. Noticeably, loss of BMD has been associated with decline in walking speed [37]. Thus, low BMD might represent an early marker of gait disturbances, and therefore of cognitive decline, in older populations.
Interestingly, the association of incident cognitive impairment with BMD in our analyses seems to be determined mainly by cortical bone density values. Approximately, 75 % of the weight of the skeleton is represented by cortical bone, which might affect our results [38]. However, it has been demonstrated that age-related bone remodeling and bone loss occur mainly at the cortical site, while trabecular bone, which was measured at the distal epiphysis, responds chiefly to mechanical stimuli [39]. Accordingly, cortical BMD has been associated with the levels of routine physical activity, which in turn is known to be associated with cognitive performance [40]. Cortical bone is mainly involved in overall bone strength and is more sensitive than trabecular bone as a marker of adverse outcomes, such as incident fractures [39].
Furthermore, cortical, but not trabecular bone density is associated with higher levels of parathyroid hormone; on the other hand, increased parathyroid hormone levels have been linked with cognitive impairment and dementia [41]. In addition, cortical bone parameters are affected by caloric restriction [38]; however, at the time of the first diagnosis, the body mass index of subjects with dementia was significantly lower.
Periostin is a matricellular protein that mediates adaptive response of cortical bone to loading; periostin deficiency has significant negative consequences on bone materials properties, damage accumulation and repair, including local modeling/remodeling in response to fatigue; at the same time, periostin exerts a neuroprotective effect and enhances cortical plasticity [42].
Also, cortical, rather than trabecular bone is influenced by renal dysfunction, which in turn is associated with cognitive impairment [43]. Nevertheless, in the present study, the association between cortical BMD and cognitive dysfunction persisted after adjusting for renal function.
The feasibility of interventions aiming at preventing cognitive impairment, or at least slowing its progression, is receiving increasing support from clinical and experimental studies on the effect of blood pressure control, inhibition of systemic or brain renin–angiotensin system, as well as vitamin D supplementation on cognitive performance and decline. However, the prerequisite for any preventive intervention is targeting individuals at higher risk of cognitive decline. Older women with reduced BMD might represent higher-risk subjects in whom early preventive interventions might be characterized by a reduced number needed to treat, and thus higher cost-effectiveness; however, the efficacy and cost/effectiveness of early interventions for the prevention of cognitive impairment in targeted populations with reduced BMD should be verified by dedicated trials.
Strengths and Limitations
The study has been conducted in a large, unselected rural Italian population of community-dwelling elderly women; these subjects generally followed a Mediterranean diet, which probably lowered their risk of both osteoporosis and cognitive impairment [44, 45]. Thus, results of this study might not apply to other populations; however, nor the Mediterranean Diet Score nor the polyphenols excretion were associated with incident cognitive impairment in our study. Also, cognitive assessment included both the MMSE and the TMT, which adds information on visuospatial scanning, sequential processing, motor speed, attention, and executive function. However, other specific cognitive domains (such as those relative to language function) were not fully explored in this study. Eventually, in our study the assessment of BMD was performed by pQCT, which is difficult to be adopted in a routine clinical setting. On the other hand, this method has proven effective in distinguishing the trabecular from cortical bone; also, its predictive power for incident fractures is similar, or even higher, as compared with DeXA.
Conclusion
Older women with reduced BMD are at higher risk of cognitive decline; the diagnostic workup in older women with osteoporosis should therefore include the assessment and monitoring of cognitive function.
Acknowledgments
The InCHIANTI study baseline (1998–2000) was supported as a targeted project (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-Up 1 (2001–2003) was funded by the U.S. National Institute on Aging, National Institutes of Health, Baltimore, Maryland (Contracts N.1-AG-1-1 and N.1-AG-1-2111). None of the sponsoring institutions interfered with the design, methods, subject recruitment, data collections, analysis, and preparation of paper.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest in this study.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent The Italian National Research Council on Aging Ethical Committee ratified the study protocol and the informed consent was obtained from all individual participants included in the study. The present study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
References
- 1. Chang KH, Chung CJ, Lin CL, Sung FC, Wu TN, Kao CH (2014) Increased risk of dementia in patients with osteoporosis: a population-based retrospective cohort analysis. Age (Dordr) 36:967–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poblador-Plou B, Calderón-Larrañaga A, Marta-Moreno J, Hancco-Saavedra J, Sicras-Mainar A, Soljak M, Prados-Torres A (2014) Comorbidity of dementia: a cross-sectional study of primary care older patients. BMC Psychiatry 14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.http://www.iofbonehealth.org/epidemiology. Accessed 25 Feb 2015)
- 4.Lin CH, Chang WC, Kuo CN, Yu HC, Yang CC, Lin YW, Hung KS, Chang WP (2015) A population-based five-year study on the risk of stroke in patients with osteoporosis in Taiwan. Bone 72(9–1):3. [DOI] [PubMed] [Google Scholar]
- 5.Loskutova N, Honea RA, Vidoni ED, Brooks WM, Burns JM (2009) Bone density and brain atrophy in early Alzheimer’s disease. J Alzheimers Dis 18:777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minn YK, Suk SH, Do SY (2014) Osteoporosis as an independent risk factor for silent brain infarction and white matter changes in men and women: the PRESENT project. Osteoporos Int 25:2465–2469 [DOI] [PubMed] [Google Scholar]
- 7.Zhou R, Zhou H, Rui L, Xu J (2014) Bone loss and osteoporosis are associated with conversion from mild cognitive impairment to Alzheimer’s disease. Curr Alzheimer Res 11:706–713 [DOI] [PubMed] [Google Scholar]
- 8.Tan ZS, Seshadri S, Beiser A, Zhang Y, Felson D, Hannan MT, Au R, Wolf PA, Kiel DP (2005) Bone mineral density and the risk of Alzheimer disease. Arch Neurol 62:107–111 [DOI] [PubMed] [Google Scholar]
- 9.Nam HS, Kweon SS, Choi JS, Zmuda JM, Leung PC, Lui LY, Hill DD, Patrick AL, Cauley JA (2013) Racial/ethnic differences in bone mineral density among older women. J Bone Miner Metab 31:190–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM (2000) Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 48:1618–1625 [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR (1975) “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198 [DOI] [PubMed] [Google Scholar]
- 12.Molloy DW, Alemayehu E, Roberts R (1991) Reliability of a Standardized Mini-Mental State Examination compared with the traditional Mini-Mental State Examination. Am J Psychiatry 148:102–105 [DOI] [PubMed] [Google Scholar]
- 13.Army Individual Test Battery Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office; 1944 [Google Scholar]
- 14.Lezak MD (1995) Neuropsychological assessment, vol 3 Oxford University Press, New York [Google Scholar]
- 15.Corrigan JD, Hinkeldey NS (1987) Relationships between parts A and B of the Trail Making Test. J Clin Psychol 43:402–408 [DOI] [PubMed] [Google Scholar]
- 16.Sievänen H, Koskue V, Rauhio A, Kannus P, Heinonen A, Vuori I (1998) Peripheral quantitative computed tomography in human long bones: evaluation of in vitro and in vivo precision. J Bone Miner Res 13:871–882 [DOI] [PubMed] [Google Scholar]
- 17.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F (1997) Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol 26(Suppl 1):S152–S160 [DOI] [PubMed] [Google Scholar]
- 18.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D (2003) Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 348:2599–2608 [DOI] [PubMed] [Google Scholar]
- 19.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME (1995) The Women’s Health and Aging Study Health and social characteristics of older women with disability. National Institute on Aging, Bethesda [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619 [DOI] [PubMed] [Google Scholar]
- 21.WHO Collaborating Centre for Drug Statistics Methodology.“ATC/DDD Methodology: History”
- 22.Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401 [Google Scholar]
- 23.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies of illness in the aged. the index of adl: a standardized measure of biological and psychosocial function. JAMA 185:914–919 [DOI] [PubMed] [Google Scholar]
- 24.Laudisio A, Marzetti E, Pagano F, Bernabei R, Zuccalà G (2009) Haemoglobin levels are associated with bone mineral density in the elderly:a population-based study. Clin Rheumatol 28:145–151 [DOI] [PubMed] [Google Scholar]
- 25.Laudisio A, Marzetti E, Antonica L, Cocchi A, Bernabei R, Zuccalà G (2008) Association of left ventricular function with bone mineral density in older women: a population-based study. Calcif Tissue Int 82:27–33 [DOI] [PubMed] [Google Scholar]
- 26.Zamboni V, Cesari M, Zuccalà G, Onder G, Woodman RC, Maraldi C, Ranzini M, Volpato S, Pahor M, Bernabei R (2006) Anemia and cognitive performance in hospitalized older patients: results from the GIFA study. Int J Geriatr Psychiatry 21:529–534 [DOI] [PubMed] [Google Scholar]
- 27.Zuccalà G, Marzetti E, Cesari M, Lo Monaco MR, Antonica L, Cocchi A, Carbonin P, Bernabei R (2005) Correlates of cognitive impairment among patients with heart failure: results of a multicenter survey. Am J Med 118:496–502 [DOI] [PubMed] [Google Scholar]
- 28.Wnuk A, Korol DL, Erickson KI (2012) Estrogens, hormone therapy, and hippocampal volume in postmenopausal women. Maturitas 73:186–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang ZD, Yu J, Zhang Q (2013) Effects of raloxifene on cognition, mental health, sleep and sexual function in menopausal women: a systematic review of randomized controlled trials. Maturitas 75:341–348 [DOI] [PubMed] [Google Scholar]
- 30.Samaras N, Samaras D, Frangos E, Forster A, Philippe J (2013) A review of age-related dehydroepiandrosterone decline and its association with well-known geriatric syndromes: is treatment beneficial? Rejuvenation Res 16:285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valenti G, Ferrucci L, Lauretani F, Ceresini G, Bandinelli S, Luci M, Ceda G, Maggio M, Schwartz RS (2009) Dehydroepiandrosterone sulfate and cognitive function in the elderly: the InCHIANTI study. J Endocrinol Invest 32:766–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedone C, Scarlata S, Napoli N, Lauretani F, Bandinelli S, Ferrucci L, Antonelli Incalzi R (2013) Relationship between bone cross-sectional area and indices of peripheral artery disease. Calcif Tissue Int 93:508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F (2013) Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc 14:877–882 [DOI] [PubMed] [Google Scholar]
- 34.Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PH, Fried L, Kestenbaum BR, Kuller LH, Langa KM, Lopez OL, Kos K, Soni M, Llewellyn DJ (2014) Vitamin D and the risk of dementia and Alzheimer disease. Neurology 83:920–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoofs MW, van der Klift M, Hofman A, van Duijn CM, Stricker BH, Pols HA, Uitterlinden AG (2004) ApoE gene polymorphisms, BMD, and fracture risk in elderly men and women: the Rotterdam study. J Bone Miner Res 19:1490–1496 [DOI] [PubMed] [Google Scholar]
- 36.Verghese J, Annweiler C, Ayers E et al. (2014) Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology 83:718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon J, Suzuki T, Yoshida H, Kim H, Yoshida Y, Iwasa H, Sugiura M, Furuna T (2007) Association between change in bone mineral density and decline in usual walking speed in elderly community-dwelling Japanese women during 2 years of followup. J Am Geriatr Soc 55:240–244 [DOI] [PubMed] [Google Scholar]
- 38.Ahn H, Seo DH, Kim HS, Choue R (2014) Calorie restriction aggravated cortical and trabecular bone architecture in ovariectomy-induced estrogen-deficient rats. Nutr Res 34:707–713 [DOI] [PubMed] [Google Scholar]
- 39.Dennison EM, Jameson KA, Edwards MH, Denison HJ, Aihie Sayer A, Cooper C (2014) Peripheral quantitative computed tomography measures are associated with adult fracture risk: the Hertfordshire Cohort Study. Bone 64:13–17 [DOI] [PubMed] [Google Scholar]
- 40.Coupaud S, McLean AN, Purcell M, Fraser MH, Allan DB (2015) Decreases in bone mineral density at cortical and trabecular sites in the tibia and femur during the first year of spinal cord injury. Bone 74:69–75 [DOI] [PubMed] [Google Scholar]
- 41.Lourida I, Thompson-Coon J, Dickens CM, Soni M, Kuźma E, Kos K, Llewellyn DJ (2015) Parathyroid hormone, cognitive function and dementia: a systematic review. PLoS One 10:e0127574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsunaga E, Nambu S, Oka M, Tanaka M, Taoka M, Iriki A (2015) Periostin, a neurite outgrowth-promoting factor, is expressed at high levels in the primate cerebral cortex. Dev Growth Differ 57:200–208 [DOI] [PubMed] [Google Scholar]
- 43.Nickolas TL, Stein EM, Dworakowski E, Nishiyama KK, Komandah-Kosseh M, Zhang CA, McMahon DJ, Liu XS, Boutroy S, Cremers S, Shane E (2013) Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res 28:1811–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero Pérez A, Rivas Velasco A (2014) Adherence to Mediterranean diet and bone health. Nutr Hosp 29:989–996 [DOI] [PubMed] [Google Scholar]
- 45.Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martínez-González MÁ, Martínez-Lapiscina EH, Fitó M, Pérez-Heras A, Salas-Salvadó J, Estruch R, Ros E (2015) Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med 175:1094–1103 [DOI] [PubMed] [Google Scholar]