Short abstract
Background
Measuring bone mineral density (BMD) around acetabular prosthetic components with computed tomography (CT) is challenged by the complex anatomy and metal artifacts. Three-dimensional (3D) segmentation is required for the analysis, but it is usually not practically applicable on current CT workstations
Purpose
To test the between-scan agreement and reliability of custom segmentation software for BMD measurements adjacent to cemented and uncemented acetabular cups in dual-energy CT (DECT).
Material and Methods
Twenty-four male patients with total hip arthroplasty were scanned and rescanned using 130-keV virtual monochromatic DECT images. Hemispherical regions of interest were defined slice-by-slice and BMD was calculated around the acetabular cup using custom segmentation software.
Results
In the uncemented cup, the mean BMD was 153 mg/cm3 with a between-scan difference of 10 mg/cm3 (P < 0.0001). In the cemented cup, the mean BMD was 186 mg/cm3 with a between-scan difference of 6 mg/cm3 (P = 0.15). In both uncemented and cemented cups the intraclass correlation coefficient between repeated measurements was >0.95 and narrow Bland–Altman Limits of Agreement.
Conclusion
BMD can be measured with high absolute between-scan agreement and good reliability adjacent to acetabular cemented and uncemented cups using DECT and segmentation software.
Keywords: Segmentation, computed tomography bone density, dual energy computed tomography, bone loss, hip loosening cup
Background
Bone loss around acetabular cups in total hip arthroplasty (THA) is considered a predictor of aseptic loosening of components (1,2) and thus reliable density measurements are crucial in the monitoring of bone loss and for research purposes. Measuring bone mineral density (BMD) around acetabular prosthetic components with computed tomography (CT) is challenged by the complex anatomy and metal artifacts. Three-dimensional (3D) segmentation with subsequent quantitative analysis is required, but it is usually not practically applicable on current CT workstations, except in spherical or box-shaped regions of interest (ROIs). Dual-energy CT (DECT) with virtual monochromatic imaging can reduce beam hardening artifacts (4–7) and thus measure attenuation more consistently than single-energy CT (3). Therefore, density measurements derived from attenuation measurements will also be more consistent. A recent phantom study by von Hamersvelt et al. (8) demonstrated measurement errors < 6% when density measurements were compared to known densities using a DECT scanner. BMD measured by CT has been examined in previous studies (1,2,9–14), but only within-scan reliability was assessed and thus measurement error induced by repeated positioning and re-scanning was not accounted for in those studies. No clinical studies have assessed between-scan reliability in the acetabulum, but one study focused on femoral and vertebral BMD (15) and another focused solely on the femoral side (16). Furthermore, the previous studies did not evaluate BMD solely in close proximity to the prostheses but used combined ROIs with bone tissue near and farther away from the metal (9–11,13,17–21). The interface between bone and cup is different in cemented and uncemented cups because the uncemented cup has a perfect hemispherical shape while the cement mantle surrounding cemented cups is more irregular which might affect BMD measurements.
The purpose of the study was to test the between-scan agreement and reliability of BMD measurements adjacent to cemented and uncemented acetabular cups using segmentation software and DECT.
Material and Methods
The sample size calculation was performed using a minimal relevant BMD difference level of 5% (10), i.e. 15 mg/cm3, 80% power, significance level of 0.05, and standard deviation (SD) estimates from a previous cadaveric study (SD = 15.2 mg/cm3) (22). Eleven patients were needed to detect the desired differences in the cemented and uncemented cup, respectively, and we included an additional patient in each group to increase power.
The inclusion criteria were: men aged 60–80 years who had received cemented or uncemented THA surgery due to primary hip arthrosis. Patients with bilateral THA, previous hip surgery, screw fixation of the acetabular cup, or primary/secondary tumors affecting the acetabulum were excluded.
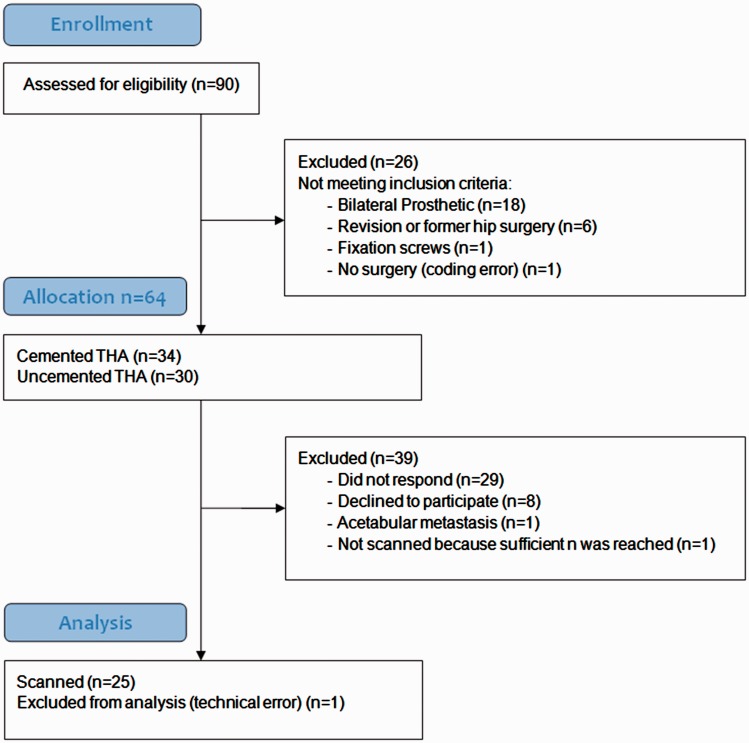
We identified 90 eligible men who had THA surgery between January 2014 and May 2016 and recruited 24 patients with uncemented or cemented acetabular hip prosthetics. Detailed inclusion and exclusion procedures can be found in Fig. 1. The patients were scanned during May to August 2016.
Fig. 1.
Enrollment procedure.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was obtained from The Regional Scientific Ethical Committee for Southern Denmark (reference no. S-20160004). Informed consent was obtained from all individual participants included in the study. According to the Danish Act on Processing of Personal Data (Act No. 429 of 31 May 2000) the study was reported to and approved by the Danish Data Protection Agency (reference no. 16/475).
Scan procedure
All scans were performed using a GE Discovery CT750 HD 64-channel scanner (GE Healthcare, Waukesha, WI, USA). The DECT protocol “GSI 1” (kVp alternating between 80 and 140 kVp, 630 mAs, rotation time of 0.5 s, 0.984 pitch, filtered back projection reconstruction and the “DETAIL” kernel) was used. The collimation was 40 mm and the “Body” bowtie filter was used. The protocol had a CT dose index (CTDI) = 17.77 mGy (32 cm phantom) which translated into a dose-length product of 295.56 mGy•cm. No additional image filtering was used. The patients were positioned feet first with no restriction to hip rotation. All scans were acquired with a slice thickness of 0.625 mm, 350 mm field of view, and an in-plane pixel size of 0.68 mm. The scans were performed with a MINDWAYS® QCT PRO (Mindways Software Inc., Austin, TX, USA) calibration phantom beneath the patients. When ROIs are drawn in each of the phantom’s calibration rods, BMD can be calculated in any ROI in the image. To reduce radiation dose, the scan length was fixed at 100 mm and covered only the acetabular cup and the anatomy approximately 25 mm above and below the cup. Thus, we did not perform a full pelvic scan as is usually done in clinical practice. After the first sequence, the patient was mobilized and walked around for approximately 1 min and then repositioned for a repeated scan in order to achieve double measurements. Virtual monochromatic images were automatically reconstructed at 130 keV without slice overlap directly from the scanner. The keV level was chosen to ensure a sufficiently high energy level to reduce beam hardening artifacts. In other scanners, 130 keV has previously been shown optimal in presence of metal implants (23,24).
Measurements
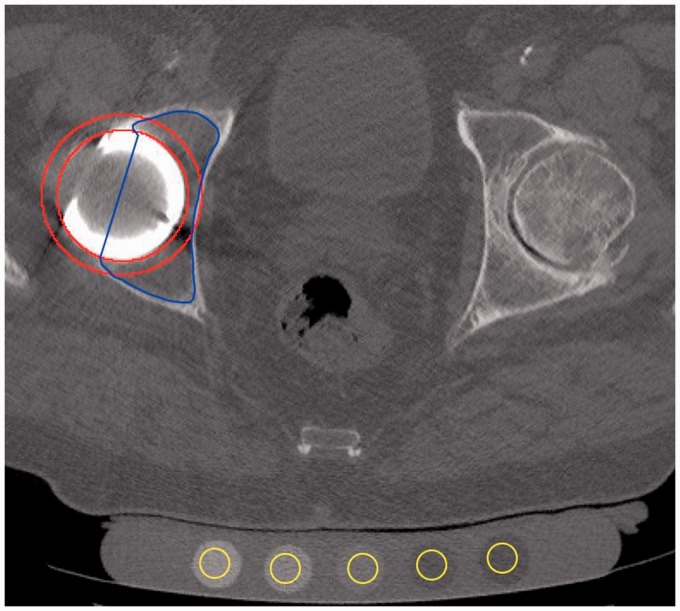
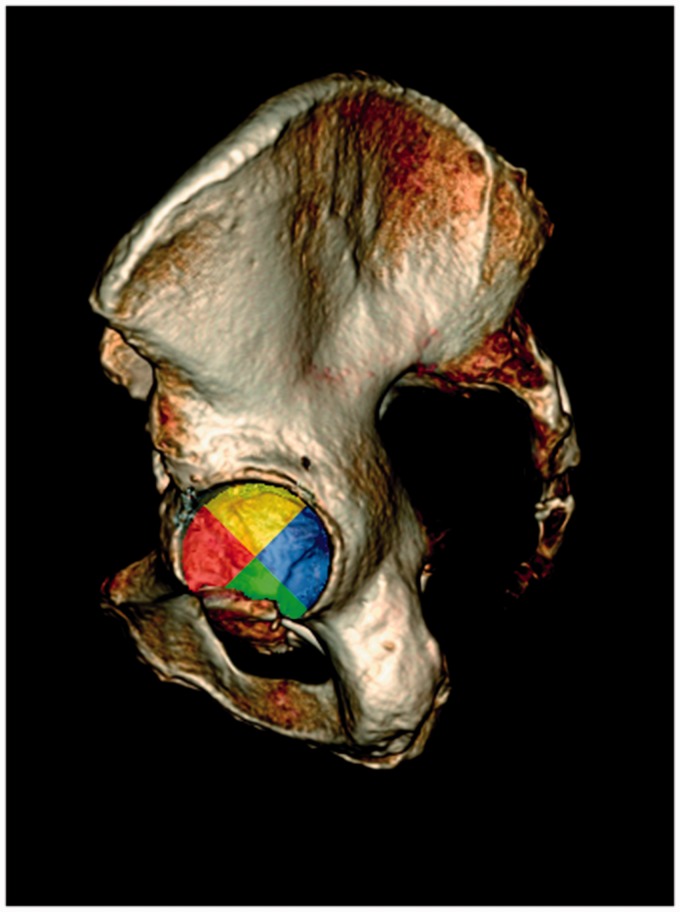
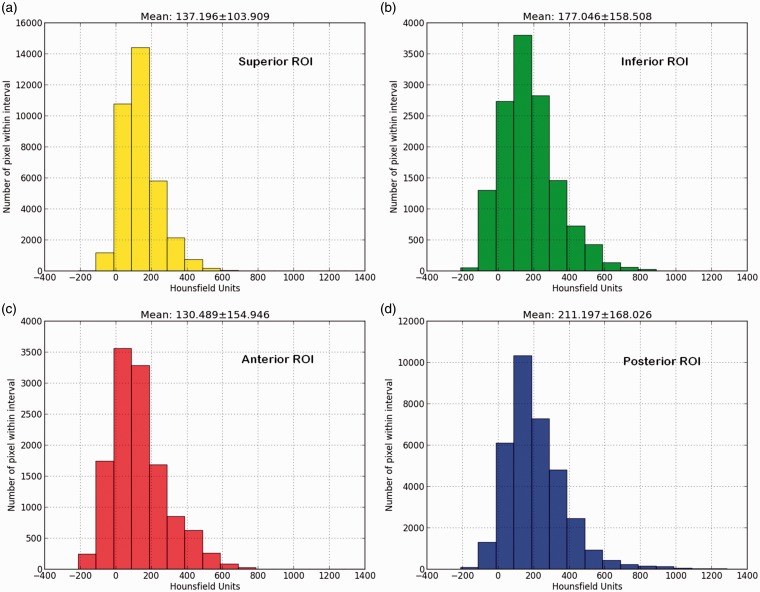
Cancellous BMD was measured in the initial scan and in the repeated scan. In each scan, a 3D hemispherical ROI in close proximity to the acetabular cup was defined using an in-house Fiji plug-in. Fiji is an open-source software platform for image analysis built on ImageJ (25). With the plug-in, the ROI could be built using slice-by-slice free-hand drawings using a ROI thickness of 5 mm and 2 mm distance to the cup (Fig. 2). The cortical bone and cement were excluded from the drawings by hand drawing. The ROI was aligned with the cup edge and sub-divided into four quadrants by defining the anteversion and inclination of the cup using reformatted coronal and sagittal images as reference (Fig. 3). Using an in-house custom written Python script (version 2.7.10, Python Software Foundation, Beaverton, OR, US), histograms with mean attenuation in each ROI and each quadrant measured in HU were generated and subsequently manually converted to BMD (Fig. 4) using a Microsoft Excel spreadsheet provided by the manufacturer of the conversion phantom. Median time between ROI drawings in the initial and repeated scans was seven days (range = 3–30 days). The drawings were performed by the first author who has > 20 years of experience in radiographic image analysis. The observer was blinded to the first ROI when drawing the repeated ROI.
Fig. 2.
Axial cross-section used for ROI definition in the right acetabulum. The ROI was defined as the intersection between the inner and outer border of the red circles and the blue free-hand area. The MINDWAYS calibration phantom is placed beneath the patient with 12-mm ROIs manually positioned in the calibration rods (yellow).
Fig. 3.
Volume-rendered pelvic CT image with superimposed color-coded ROI segments corresponding to the histograms in Fig. 4.
Fig. 4.
Example of histograms showing the distribution of attenuation values in HU for each ROI in a patient: (a) superior ROI; (b) inferior ROI; (c) anterior ROI; and (d) posterior ROI. Mean attenuation and SD in the ROIs are noted above each histogram.
Statistics
All variables were continuous and are summarized by mean, number of observations, and 95% confidence intervals (95% CI). Data were determined normally distributed using the Shapiro–Wilk test. We used mixed effects regression modelling, using measurement as fixed effect to estimate the differences between repeated BMD measurements. The absolute agreement between the repeated measurements was analyzed with Bland–Altman plots (26) including mean difference and limits of agreement while the between-scan reliability was assessed by intraclass correlation coefficients (ICC) based on two-way random effects models (27,28). Furthermore, repeatability coefficients (RC) and corresponding 95% CI were calculated for the repeated measurements according to Bartlett and Frost (28). RC is the difference below which the real difference will lie with 95% probability. The RC was compared with the minimal relevant difference by converting to percent by dividing RC with the mean BMD*100. P values < 0.05 were considered statistically significant. All analyses were performed using STATA/SE 14.0 (StataCorp. LP, College Station, TX, USA). The study is reported in accordance with the Guidelines for Reporting Reliability and Agreement Studies (GRASS) (29).
Results
In the cemented group (n = 12), the median age was 74.5 years (age range = 70–80 years) with a median time from surgery to scan of 9.5 months (range = 3–15 months). In the uncemented group (n = 12), the median age was 66.5 years (age = range 62–70 years) with a median time from surgery to scan of 12 months (range = 8–32 months). Mean cup size was 56 mm (range = 52–62 mm).
The results regarding absolute agreement of the repeated BMD measurements are summarized in Table 1. For all acetabular quadrants combined around the uncemented cup, the mean BMD was 153 mg/cm3 with a between-scan difference of 10 mg/cm3 (P < 0.0001) and an RC of 18 mg/cm3. In the cemented cup, the mean BMD was 186 mg/cm3 with a between-scan difference of 6 mg/cm3 (P = 0.15) and an RC of 29 mg/cm3.
Table 1.
Mean BMD and difference between repeated BMD measurements in uncemented and cemented cups (units of mg K2HPO4/cm3).
|
Volume of interest |
Mean BMD measurement 1 | Mean BMD measurement 2 | Difference (%) | 95% CI | P value |
|---|---|---|---|---|---|
| Uncemented cup (n = 12) | |||||
| All quadrants | 153 | 163 | 10 (6.3) | 4–15 | <0.0001 |
| Superior quadrant | 164 | 172 | 8 (4.8) | −0.2–16 | 0.06 |
| Inferior quadrant | 221 | 220 | −1 (0.5) | −11–10 | 0.88 |
| Anterior quadrant | 148 | 148 | 0 (0.0) | −12–12 | 0.99 |
| Posterior quadrant | 127 | 153 | 26 (18.6) | −7–59 | 0.13 |
| Cemented cup (n = 12) | |||||
| All quadrants | 186 | 192 | 6 (3.2) | −2–14 | 0.15 |
| Superior quadrant | 233 | 250 | 17 (7.0) | 3–32 | 0.02 |
| Inferior quadrant | 133 | 128 | −5 (3.8) | −33–23 | 0.71 |
| Anterior quadrant | 74 | 88 | 14 (17.3) | −16–45 | 0.36 |
| Posterior quadrant | 195 | 191 | −4 (2.1) | −20–11 | 0.57 |
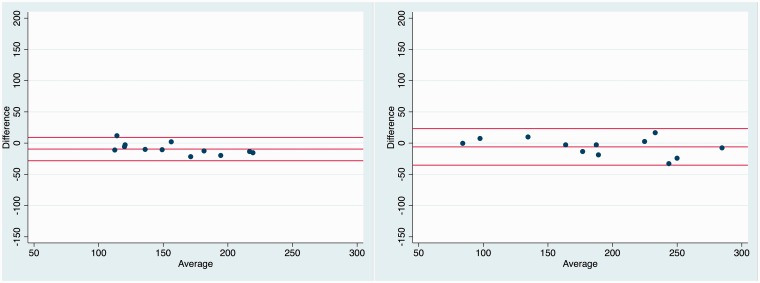
In the sub-segmented ROIs, we found no significant differences in the uncemented cup, but in the cemented cup a statistically significant between-scan difference of 17 mg/cm3 (P = 0.02) was found in the superior ROI (Table 2). The Bland–Altman Limits of Agreement in the uncemented cup were narrower than in the cemented cup (Fig. 5).
Table 2.
Repeatability coefficients (RC) in units of mg/cm3 in uncemented and cemented cups with corresponding 95% CI. The percentage compares RC to the mean Bone Mineral Density.
| Volume of interest | RC (%) | 95% CI |
|---|---|---|
| Uncemented cup (n = 12) | ||
| All quadrants | 18 (11) | 12–28 |
| Superior quadrant | 28 (17) | 18–42 |
| Inferior quadrant | 36 (16) | 24–55 |
| Anterior quadrant | 42 (28) | 27–63 |
| Posterior quadrant | 115 (82) | 75–174 |
| Cemented cup (n = 12) | ||
| All quadrants | 29 (15) | 19–43 |
| Superior quadrant | 50 (50) | 33–75 |
| Inferior quadrant | 97 (97) | 64–148 |
| Anterior quadrant | 106 (131) | 70–162 |
| Posterior quadrant | 53 (27) | 35–81 |
The percentage compares RC to the mean BMD.
Fig. 5.
Bland–Altman plots with average BMD (x-axis), mean difference, and limits of agreement (y-axis) in the uncemented cup (left) and cemented cup (right) (n = 24).
In the combined ROI, the reliability expressed as ICC was > 0.95 in both cup types (Table 3).
Table 3.
ICCs and 95% CI in uncemented and cemented cups derived from DECT.
| Volume of interest | ICC | 95% CI |
|---|---|---|
| Uncemented cup (n = 12) | ||
| All quadrants | 0.95 | 0.56–0.99 |
| Superior quadrant | 0.94 | 0.79–0.98 |
| Inferior quadrant | 0.98 | 0.92–0.99 |
| Anterior quadrant | 0.95 | 0.84–0.99 |
| Posterior quadrant | 0.62 | 0.14–0.87 |
| Cemented cup (n = 12) | ||
| All quadrants | 0.97 | 0.90–0.99 |
| Superior quadrant | 0.88 | 0.56–0.97 |
| Inferior quadrant | 0.92 | 0.75–0.98 |
| Anterior quadrant | 0.72 | 0.29–0.91 |
| Posterior quadrant | 0.94 | 0.81–0.98 |
Discussion
This is, to our knowledge, the first study to assess between-scan reliability of acetabular BMD measurements with CT in vivo. The results demonstrate that BMD can be assessed adjacent to acetabular hip prosthetics using segmentation software and DECT.
Measurement error
The mean BMD difference between the measurements in the combined ROI was well below or close to the predefined 5% minimal relevant difference. However, the measurement uncertainty expressed by RC was 11% in the uncemented and 15% in the cemented cup in the combined ROI and as much as 16–130% in some of the sub-segmented ROIs. This may be explained by the small number of pixels in each quadrant. In the uncemented cup, the most frequent occurrence of bone loss is the supra-acetabular ileum corresponding to the superior ROI (30). In this ROI, the measurement uncertainty was 17% and 21%, reflecting a substantial measurement error which makes the present method less useful in the sub-segmented ROIs. The clinical significance of periprosthetic bone loss is still unclear and thus the minimal relevant difference is arbitrary. However, regular X-ray cannot detect bone loss < 30% and X-ray only provides two-dimensional images. Thus, CT may be clinically advantageous compared to X-ray.
The results are in line with previous ex vivo findings (22). Not surprisingly, the results suggest that the free-hand drawing is more difficult to replicate in a clinical setting. The ex vivo study used young porcine specimens with no degenerative bone changes and, in the cemented cup, with no or little osseo-integration of the cement mantle; thus, the study is strengthened by the in vivo design with double measurements. Because single-slice measurement approaches are prone to patient-positioning error, the current study is also strengthened by the use of volumetric measurements. Finally, the observer was blinded to the first ROI positioning, which would not be the case in a clinical setting or in a longitudinal study. Thus, we expect that the repeatability would be better with no blinding.
The study also has some limitations. Even though we included a sufficient number of participants, this is still a small-scale study sensitive to outliers. This is markedly reflected in the high RCs in the sub-segmented ROIs, which furthermore include relatively few pixels and are thus more sensitive to differences in the free-hand ROI positioning compared to the combined ROI. Another limitation is the fact that because the scans were performed at the same occasion with very short time between the repeated scans, the study does not consider that the measurements may vary from day to day despite air calibration. However, using two subsequent scans also opens the possibility of assessing the pure measurement error caused by patient positioning and random fluctuations in CT number measurements because no actual changes in BMD could have occurred given the short time frame.
No female participants were included and thus the BMD in the current study is probably higher than the population mean because women are more prone to osteoporosis. However, the Bland–Altman plots did not reveal any signs of dependency of density in the measurements.
In the current study, only a few different cup types were included and thus the effect of cup types was neglected. However, because we only focused on differences between repeated measurements and all cups were titanium-backed cups with polyethylene liners or cemented polyethylene cups and the stems had 32-mm steel heads, we do not believe this to be relevant.
The study demonstrates that it is crucial to assess measurement error. Even though we found very small mean between-scan differences, there were large individual differences when the acetabular ROI was divided into smaller segments. Thus, the software may be useful for research at group level, while clinical use with individual monitoring over time is not appropriate with this method.
In conclusion, BMD can be measured with high absolute between-scan agreement and good reliability adjacent to acetabular cemented and uncemented cups using DECT and segmentation software. However, the measurement error must be considered, especially when smaller segments of the bone are assessed.
Acknowledgments
The authors greatly appreciate the statistical advice from biostatistician Oke Gerke, Department of Clinical Physiology and Nuclear Medicine, Odense University Hospital. The authors are also grateful for the assistance of project nurse Annie Gam-Pedersen, Department of Orthopaedic Surgery and Traumatology, Odense University Hospital. Furthermore, they thank Peter Traise, Medical Imaging Department, Bathurst Health Service, New South Wales, Australia, for his assistance in critically appraising the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Mueller LA, Nowak TE, Mueller LP, et al. Acetabular cortical and cancellous bone density and radiolucent lines after cemented total hip arthroplasty: a prospective study using computed tomography and plain radiography. Arch Orthop Trauma Surg 2007; 127:909–917. [DOI] [PubMed] [Google Scholar]
- 2.Stepniewski AS, Egawa H, Sychterz-Terefenko C, et al. Periacetabular bone density after total hip arthroplasty a postmortem analysis. J Arthrop. 2008; 23:593–599. [DOI] [PubMed] [Google Scholar]
- 3.Matsuda I, Akahane M, Sato J, et al. Precision of the measurement of CT numbers: comparison of dual-energy CT spectral imaging with fast kVp switching and conventional CT with phantoms. Japan J Radiol 2012; 30:34–39. [DOI] [PubMed] [Google Scholar]
- 4.Yu L, Leng S, McCollough CH. Dual-energy CT-based monochromatic imaging. Am J Roentgenol 2012; 199(Suppl. 5):9–15. [DOI] [PubMed] [Google Scholar]
- 5.Meinel FG, Bischoff B, Zhang Q, et al. Metal artifact reduction by dual-energy computed tomography using energetic extrapolation: a systematically optimized protocol. Invest Radiol 2012; 47:406–414. [DOI] [PubMed] [Google Scholar]
- 6.Boas FE, Fleischmann D. CT artifacts: causes and reduction. Imaging Med 2012; 4:21. [Google Scholar]
- 7.Filograna L, Magarelli N, Leone A, et al. Performances of low-dose dual-energy CT in reducing artifacts from implanted metallic orthopedic devices. Skeletal Radiol 2016; 45:937–947. [DOI] [PubMed] [Google Scholar]
- 8.van Hamersvelt RW, Schilham AMR, Engelke K, et al. Accuracy of bone mineral density quantification using dual-layer spectral detector CT: a phantom study. Eur Radiol 2017; 27:4351–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boomsma MF, Slouwerhof I, van Lingen C, et al. CT-based quantification of bone stock in large head metal-on-metal unilateral total hip replacements. Eur J Radiol 2016; 85:760–763. [DOI] [PubMed] [Google Scholar]
- 10.Kress AM, Schmidt R, Vogel T, et al. Quantitative computed tomography-assisted osteodensitometry of the pelvis after press-fit cup fixation: a prospective ten-year follow-up. J Bone Joint Surg Am 2011; 93:1152–1157. [DOI] [PubMed] [Google Scholar]
- 11.Looney RJ, Boyd A, Totterman S, et al. Volumetric computerized tomography as a measurement of periprosthetic acetabular osteolysis and its correlation with wear. Arthritis Res 2002; 4:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt R, Pitto R, Kress A, et al. Inter- and intraobserver assessment of periacetabular osteodensitometry after cemented and uncemented total hip arthroplasty using computed tomography. Arch Orthop Trauma Surg 2005; 125:291–297. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt R, Kress AM, Nowak M, et al. Periacetabular cortical and cancellous bone mineral density loss after press-fit cup fixation: a prospective 7-year follow-up. J Arthrop 2012; 27:1358–1363. [DOI] [PubMed] [Google Scholar]
- 14.Puri L, Wixson RL, Stern SH, et al. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am 2002; 84:609–614. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Sode M, Saeed I, et al. Automated registration of hip and spine for longitudinal QCT studies: Integration with 3D densitometric and structural analysis. Bone 2006; 38:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang TF, Keyak JH, Heitz MW, et al. Volumetric quantitative computed tomography of the proximal femur: Precision and relation to bone strength. Bone 1997; 21:101–108. [DOI] [PubMed] [Google Scholar]
- 17.Chandran P, Azzabi M, Andrews M, et al. Periprosthetic bone remodeling after 12 years differs in cemented and uncemented hip arthroplasties. Clin Orthop Rel Res 2012; 470:1431–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howie DW, Neale SD, Martin W, et al. Progression of periacetabular osteolytic lesions. J Bone Joint Surg Am 2012; 94:e1171–1176. [DOI] [PubMed] [Google Scholar]
- 19.Mueller LA, Schmidt R, Ehrmann C, et al. Modes of periacetabular load transfer to cortical and cancellous bone after cemented versus uncemented total hip arthroplasty: a prospective study using computed tomography-assisted osteodensitometry. J Orthop Res 2009; 27:176–182. [DOI] [PubMed] [Google Scholar]
- 20.Mueller LA, Voelk M, Kress A, et al. Progressive cancellous and cortical bone remodeling after press-fit cup fixation: a 3-year followup. Clin Orthop Rel Res 2007; 463:213–220. [PubMed] [Google Scholar]
- 21.Mulier M, Jaecques SVN, Raaijmaakers M, et al. Early periprosthetic bone remodelling around cemented and uncemented custom-made femoral components and their uncemented acetabular cups. Arch Orthop Trauma Surg 2011; 131:941–948. [DOI] [PubMed] [Google Scholar]
- 22.Mussmann B, Overgaard S, Torfing T, et al. Intra- and inter-observer agreement and reliability of bone mineral density measurements around acetabular cup: a porcine ex-vivo study using single- and dual-energy computed tomography. Acta Radiologica Open 2017; 6(7):2058460117719746. [DOI] [PMC free article] [PubMed]
- 23.Zhou CS, Zhao YE, Luo S, et al. Monoenergetic imaging of dual-energy CT reduces artifacts from implanted metal orthopedic devices in patients with factures. Acad Radiol 2011; 18:1252–1257. [DOI] [PubMed] [Google Scholar]
- 24.Guggenberger R, Winklhofer S, Osterhoff G, et al. Metallic artefact reduction with monoenergetic dual-energy CT: systematic ex vivo evaluation of posterior spinal fusion implants from various vendors and different spine levels. Eur Radiol 2012; 22:2357–2364. [DOI] [PubMed] [Google Scholar]
- 25.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 2007; 17:571–582. [DOI] [PubMed] [Google Scholar]
- 27.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psych Bull 1979; 86:420–428. [DOI] [PubMed] [Google Scholar]
- 28.Bartlett JW, Frost C. Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol 2008; 31:466–475. [DOI] [PubMed] [Google Scholar]
- 29.Kottner J, Audige L, Brorson S, et al. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J Clin Epidemiol 2011; 64:96–106. [DOI] [PubMed] [Google Scholar]
- 30.Stamenkov RB, Howie DW, Neale SD, et al. Distribution of periacetabular osteolytic lesions varies according to component design. J Arthrop 2010; 25:913–919. [DOI] [PubMed] [Google Scholar]