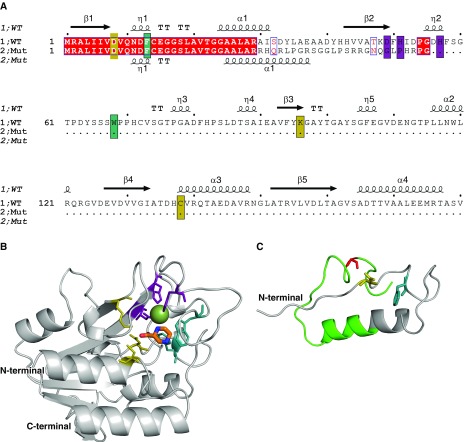
Figure 2.
Structural analysis of a novel pncA frameshift mutation. (A) The sequence alignment between the wild-type and mutant protein sequences shows that only the first 29 residues are conserved (red), and that the frameshift leads to the introduction of a premature stop codon. The catalytic triad, substrate-binding site, and iron center are highlighted in yellow, teal, and purple, respectively. The secondary structure of the wild-type PncA protein is shown above the sequence (β = β sheet, α = α helix, and η = loop). (B) The structure of the wild-type PncA protein is represented as a ribbon (gray), bound to the drug pyrazinamide (in orange segments). The Fe2+ ion is shown as a green sphere. (C) The modeled structure of the mutant PncA protein highlights that most of the catalytic site and structure of the wild-type protein is absent in the mutant. The region not conserved with the wild-type sequence is shown in green. Both wild-type and mutant structures are shown from the same perspective. Mut = mutant; WT = wild type.