Abstract
Objectives:
This open-label, randomized phase II trial evaluated antitumor efficacy of an antiestrogen, fulvestrant, in combination with human epidermal growth factor receptor (EGFR) inhibitor, erlotinib, in advanced non-small cell lung cancer (NSCLC) patients.
Materials and Methods:
Patients with advanced or metastatic NSCLC, ECOG 0–2, previous chemotherapy unless patient refusal, and no prior EGFR-directed therapy were randomized 2:1 to erlotinib 150 mg oral daily plus 500 mg intramuscular fulvestrant on day 1, 15, 29 and every 28 days thereafter or erlotinib alone 150 mg oral daily. The primary end point was objective response rate (ORR); secondary endpoints included progression free survival (PFS) and overall survival (OS).
Results:
Among 106 randomized patients, 100 received at least one dose of study drug. ORR was 16.4% (11 of 67 patients) for the combination versus 12.1% (4 of 33 patients) for erlotinib (p=0.77). PFS median 3.5 versus 1.9 months [HR=0.86, 95% CI (0.52–1.43), p=0.29] and OS median 9.5 versus 5.8 months [HR=0.92, 95% CI (0.57–1.48), p=0.74] numerically favored the combination. In an unplanned subset analysis, among EGFR wild type patients (n=51), but not EGFR mutant patients (n=17), median PFS was 3.5 versus 1.7 months [HR=0.35, 95% CI (0.14–0.86), p=0.02] and OS was 6.2 versus 5.2 months [HR=0.72, 95% CI (0.35 to 1.48), p=0.37] for combined therapy versus erlotinib, respectively. Notably, EGFR WT patients were more likely to be hormone receptorpositive (either estrogen receptor α- and/or progesterone receptor-positive) compared to EGFR mutant patients (50% versus 9.1%, respectively) (p=0.03). Treatment was well tolerated with predominant grade 1–2 dermatologic and gastrointestinal adverse effects.
Conclusion:
Addition of fulvestrant to erlotinib was well tolerated, with increased activity noted among EGFR wild type patients compared to erlotinib alone, albeit in an unplanned subset analysis.
Keywords: Erlotinib, Fulvestrant, Lung Cancer, EGFR, Estrogen, Estrogen Receptor
1. Introduction
Lung cancer is the leading cause of cancer death in the United States, responsible for more deaths each year than colon, breast, and prostate cancer combined [1]. The epidermal growth factor receptor (EGFR/ErbB1/HER1) has been implicated in lung cancer pathogenesis [2], and activating mutations of the tyrosine kinase domain of EGFR underlie the responsiveness of non-small cell lung cancer (NSCLC) to EGFR inhibitors [2–4]. Compared to standard chemotherapy, treatment with EGFR inhibitors increased progression free survival (PFS) in untreated EGFR mutant NSCLC [5, 6]. Investigation of the activity of EGFR inhibitors with other agents has been explored, but no current phase III data show superiority of an approach combining an EGFR inhibitor with a second agent [7, 8]. Understandably, there has been a focus on EGFR inhibitors in EGFR mutant NSCLC patients, but the majority of patients worldwide are EGFR wild type (EGFR WT) [9]. Recent results from randomized prospective trials have presented findings showing that most patients with EGFR WT tumors do not benefit from treatment with EGFR inhibitors alone [10] and the prescribing indication for EGFR inhibitors in NSCLC was therefore limited to first-line, maintenance, or second-line therapy in patients with metastatic disease. However, during the period of patient accrual for this study from 2006–2011, the correlation between EGFR status and treatment was not fully elucidated. Although EGFR inhibitors are no longer recommended in patients who lack EGFR driver mutations, our analysis conducted from 2006–2011 demonstrates activity of an EGFR inhibitor when combined with antiestrogen therapy among EGFR WT patients, thus providing evidence of potential cross-talk between these pathways.
An association between estrogens and lung cancer risk first emerged in the 1973 Coronary Drug Project Trial when men who had previously suffered a myocardial infarction were randomly assigned to receive either equine estrogen or placebo, with the anticipation that a decrease in cardiac events would be observed in the estrogen arm. However, the trial was stopped early when an increase in lung cancer mortality was observed in patients receiving estrogen [11]. More recent randomized, prospective studies have also reported an increase in lung cancer mortality from combined hormone use, and increased incidence of lung cancer in patients receiving a combination of estrogen plus progestin as hormone replacement therapy [12, 13]. In support of these findings, several laboratory studies suggest the role of estrogens in promoting lung carcinogenesis and progression, as well as evidence of estrogen receptor-α (ER- α) and estrogen receptor-β (ER-β) mRNA and protein expression in malignant lung epithelial cells [14–22]. In comprehensive studies, ER-α and ER-β were reported in the nucleus and cytoplasm of NSCLC cells, but the level and frequency of receptor expression varied among different antibodies used [23]. Progesterone receptor (PgR) was also reported to be expressed in the nuclei of NSCLC cells. Further, nuclear ER expression was found to correlate with adenocarcinoma histology, female gender, and history of never smoking [23]. The potential roles and expression of steroid hormone receptors, including estrogen receptor, progesterone receptor, and aromatase in NSCLC clinical outcomes have previously been presented [24–26]Notably, fulvestrant, a pure antiestrogen that downregulates ER in target tissues, is approved for treatment of progressive breast cancer, and this antiestrogen is further reported to exert significant antitumor activity in preclinical models of human NSCLC [14, 15, 22, 27–29].
Details of potential interactions between EGFR and the ER signaling pathway have also been reported [22, 25]. ER may mediate gene transcription by integrating signals from EGFR- activated pathways as well as from steroid binding [22, 25, 26, 30, 31]. Cross-communication between EGFR and ER appears to promote significant stimulation of target cell proliferation and a reduction in the apoptotic loss of those cells that express both receptor signaling pathways [32]. These observations are supported by several preclinical studies demonstrating that combinations of gefitinib or erlotinib with fulvestrant resulted in additive antitumor effects in ER-expressing NSCLC cells in vitro and in tumor xenograft models in vivo [22, 25, 28].
Gefitinib and fulvestrant were evaluated as part of a phase I trial in NSCLC. Results of the trial demonstrated a promising safety profile as well as evidence of potential antitumor activity [33]. We later initiated a phase II trial of gefitinib and fulvestrant in patients with advanced NSCLC, but the trial was suspended after four patients enrolled because further development and availability of gefitinib in the United States stopped. The trial was then amended for use of erlotinib rather than gefitinib, thereby allowing the current open-label, randomized, phase II study of patients with advanced NSCLC to evaluate erlotinib plus fulvestrant versus erlotinib alone.
2. Materials and Methods
2.1. Patients and Samples
Key inclusion criteria included age ≥18 years, pathologically-proven advanced (Stage IIIb or IV) NSCLC (AJCC 6th edition), previously treated with standard chemotherapy unless patient refusal or inability to receive chemotherapy, Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2, measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST 1.0), fresh or archival tumor tissue availability, and adequate organ function. Exclusion criteria included chemotherapy or non-cytotoxic investigational agents within 4 weeks, prior history of an EGFR inhibitor or antiestrogen for cancer treatment and active CNS metastasis.
2.2. Trial Design and Treatment
This study was sponsored by Translational Research in Oncology-US Network (NCT00100854). This multicenter, randomized, open-label phase II trial compared the objective response rate (ORR) as defined by RECIST 1.0 in the erlotinib plus fulvestrant arm versus erlotinib alone arm. Secondary objectives included PFS, OS, and characterization of toxicities and identification of patient subgroups that are distinct with respect to response.
Patients were randomized 2:1 by a random permuted block design, using a web-based system to receive oral erlotinib 150 mg oral daily plus fulvestrant 500 mg intramuscular on day 1, 15, 29 and every 28 days thereafter or erlotinib 150 mg oral daily alone. The four patients enrolled when gefitinib was the EGFR inhibitor are not included in this analysis. No dose reductions were allowed for fulvestrant, and one dose reduction (100 mg) was permitted for erlotinib. For an intolerable grade 3 or 4 rash, erlotinib was reduced to 100 mg daily when the rash resolved to less than grade 2; if possible, the original dose was reinstated if there was no further toxicity for an entire cycle at the reduced dose. Suggested measures for management of skin toxicity included topical or systemic antibiotics along with topical or a short course of systemic corticosteroids. Treatment was continued until documented progression, unacceptable toxicity, withdrawal of consent, or death.
2.3. Assessments
During the study, tumor measurement and survival status were collected for evaluation of ORR, PFS, and OS. Computed tomography (CT) scans were obtained at baseline and every two cycles (8 weeks). Patients were also monitored for adverse events (AEs), changes in laboratory values and physical examination findings on days 1 and 15 of the first cycle, day 1 of cycle 2 and 3, and day 1 of every other subsequent cycle.
2.4. Evaluation of Safety and Tolerability
Safety and tolerability were assessed from initiation of study treatment until at least 30 calendar days after the last dose of study drug. The JCCC Data Safety Monitoring Board, constituted prior to the treatment of the first patient, received quarterly reports and met at least twice yearly to review the incidence and severity of all AEs and serious AEs. Two interim analyses occurred, one for safety after 5 patients were treated with the combination therapy and another for futility (ORR ≤ 5%) in the combination arm after the 51st patient received the 8-week assessment.
2.5. Determination of EGFR Status and Tissue ER-α and PgR
Prior to cycle 1 day 1 (C1D1), tumor tissue (new or archival) was obtained for assessment of tissue biomarkers. Either formalin-fixed, paraffin-embedded tissue blocks, or unstained sections 4–5 microns thick were used with immunohistochemistry assays to assess ER-α and PgR expression in NSCLC. Tissue from patients who had not undergone EGFR mutation testing in a CLIA-certified lab was sequenced for mutations in the EGFR tyrosine kinase domain (exons 18–21) with results for exon 19 and 21 required to be considered informative results. T790M would have been detected with the targeted sequencing that was done for this study, but no T790M mutations were noted. Evaluation of nuclear ER-α using 6F11 anti-ER antibody (Abcam) and PgR using PgR636 anti-PgR antibody (DAKO) (both at 4 micrograms/ml, approximately 1:50 dilution) was performed with antigen retrieval (Tris/EDTA buffer, pH 9) in a central CLIA-certified laboratory using established immunohistochemical assays after validation of specific antibodies for ER-α and PgR in NSCLC tissue [34–36]. The distribution of primary mouse anti-receptor antibodies bound to tissue was detected by a secondary goat anti-mouse immunoglobulin conjugated to horseradish-peroxidase-labelled-dextran polymer (DAKO) and localized with diaminobenzidine. Positive and negative controls were included with each assay, which consisted of known ER-α-positive breast cancers and known PgR-positive breast cancers. Confirmatory findings with human breast cancer cell lines (MCF-7/ER-positive and T47D/PR-positive) also supported the findings. . Only freshly-cut NSCLC specimens were used for these analyses. Tumor specimens were scored by a board-certified pathologist for hormone receptor expression as either positive or negative by the pathology reviewer using the ASCO-CAP guidelines criteria [37, 38].
2.6. Determination of Blood Estrone and Estradiol
Estradiol and estrone in patient serum collected at day 1 of cycles 1–3 were measured in duplicate, using a simultaneous ultra-high performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) technique as described [39, 40]. Serum (0.5 ml), spiked with an internal standard (2,4,16,16,17-d5–17 β-estradiol) was extracted with n-butyl chloride, derivatized with dansyl chloride and transferred to glass vials for injection. Steroids were eluted from a reversed-phase column, with an acetonitrile:water (0.1% formic acid) gradient. Detection and quantitation were achieved as previously described [39]. The calibration curves range from 1pg/ml (lower limit of quantitation) to 200 pg/ml. Values less than 1 were below the level of detection and assigned a value of 0.
2.7. Statistical Design and Analyses
Patients were stratified based on gender and ECOG performance status. In a one-sided exact test, 68 patients yielded a power of 82% (α= 0.10) to detect a 100% improvement in response rate with erlotinib plus fulvestrant (expected RR=20%) compared to historical data [9, 41]. The primary end-point was ORR in each arm without calling for comparative analysis between the treatment arms. In analyzing the data, we have performed exploratory analyses comparing the two treatment arms. ORR and a variety of clinical factors present at baseline by arm were analyzed using a Chi-Square test, Fisher’s exact test, or Wilcoxon rank sum test. Median PFS and OS were estimated from Kaplan-Meier curves. Log-rank test was used to test the difference in PFS and OS between treatment arms.
3. Results
3.1. Patient Characteristics
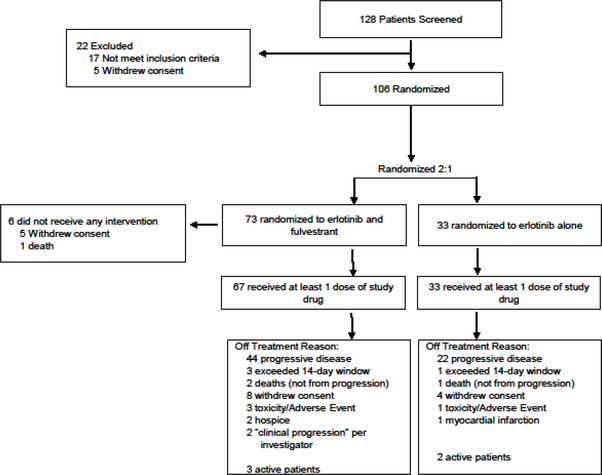
Between March 2006 and June 2011, 106 patients were randomized to receive erlotinib plus fulvestrant (n=73) or erlotinib alone (n=33). Six patients received no study therapy, for a total of 100 treated patients—67 patients in erlotinib plus fulvestrant arm and 33 patients in erlotinib alone arm (Figure 1). Baseline characteristics were well balanced between the randomized treatment groups (Table 1).
Figure 1.
CONSORT diagram.
Table 1.
Baseline characteristics
| Patient Characteristic | Erlotinib and Fulvestrant (n=67) |
Erlotinib Alone (n=33) |
P value | |
|---|---|---|---|---|
| Median Age (years) (SD) | 68 (11) | 66 (12) | 0.898 | |
| Gender | 0.728 | |||
| Female (%) | 39 (58) | 18 (55) | ||
| Male (%) | 28 (42) | 15 (45) | ||
| Ethnicity | 0.615 | |||
| Asian (%) | 17 (25) | 7 (21) | ||
| Black (%) | 3 (4) | 2 (6) | ||
| Hispanic (%) | 5 (7) | 5 (15) | ||
| White (%) | 42 (63) | 19 (58) | ||
| Missing (%) | 0 (0) | 0 (0) | ||
| ECOG | 0.787 | |||
| 0 (%) | 27 (40) | 13 (39) | ||
| 1 (%) | 33 (49) | 15 (45) | ||
| 2 (%) | 7 (10) | 5 (15) | ||
| Smoking Status | 0.619 | |||
| Yes (%) | 49 (74) | 26 (79) | ||
| No (%) | 17 (26) | 7 (21) | ||
| Missing (%) | 1 (1) | 0 (0) | ||
| EGFR | 0.119 | |||
| Mutant (%) | 10 (15) | 7 (21) | ||
| Wild Type (%) | 39 (58) | 12 (36) | ||
| Not done (%) | 18 (27) | 14 (43) | ||
| Histology | 0.931 | |||
| Adenocarcinoma (%) | 41 (61) | 21 (64) | ||
| Squamous (%) | 13 (19) | 6 (18) | ||
| Other (%) | 3 (4) | 2 (6) | ||
| Missing (%) | 10 (15) | 4 (12) | ||
| Stage at Enrollment | 0.095 | |||
| IIIB (%) | 10 (15) | 1 (3) | ||
| IV (%) | 57 (85) | 32 (97) | ||
| Number of Prior Treatments for Stage IV | 0.574 | |||
| 0 (%) | 29 (43) | 11 (33) | ||
| 1 (%) | 21 (31) | 10 (30) | ||
| 2 (%) | 11 (16) | 7 (21) | ||
| 3 (%) | 2 (3) | 2 (6) | ||
| 4 (%) | 2 (3) | 0 (0) | ||
| Missing (%) | 2 (3) | 3 (9) | ||
| Cycles Completed (Mean) (SD) | 5.1 (7.2) | 4.4 (7.2) | 0.646 | |
3.2. Efficacy
ORR was 16.4% for erlotinib plus fulvestrant and 12.1% for erlotinib alone (p=0.77). Among 68 patients for whom EGFR status could be assessed, 17 patients had mutations and 51 were EGFR WT. Most EGFR mutant patients responded to therapy without clear differences between the two treatment arms in this small subset. For patients with EGFR WT, clinical benefit rate (CBR), defined as stable disease and partial response, was 41% (16 of 39) in the erlotinib plus fulvestrant arm and 8.3% (1 of 12) in the erlotinib alone arm (p=0.04) (Table 2).
Table 2.
Response rates among combination erlotinib and fulvestrant versus erlotinib alone
| Erlotinib and Fulvestrant |
Erlotinib Alone |
*P value | ||
|---|---|---|---|---|
| All Patients | ||||
| ORR (%) | 11 (16.4) | 4(12.1) | 0.77 | |
| PR (% | 11 (16.4) | 4(12.1) | ||
| SD (%) | 18 (27) | 7 21) | ||
| PD (%) | 25 (37) | 16 (46) | ||
| NA (%) | 13 (19) | 6 (18) | ||
| Total | 67 | 33 | ||
| EGFR Wild Type | ||||
| ORR (%) | 2(5.1) | 0 (0) | >0.99 | |
| PR (% | 2 (5.1) | 0 (0) | ||
| SD (%) | 14 (36) | 1(8) | ||
| PD (%) | 15 (38) | 11 (92) | ||
| NA (%) | 8 (21) | 0 (0) | ||
| Total | ||||
| ORR (%) | 3 (7.7) | 0 (0) | >0.99 | |
| PR (% | 3 (7.7) | 0 (0) | ||
| SD (%) | 14 (36) | 1 (8) | ||
| PD (%) | 15 (38) | 11 (92) | ||
| NA (%) | 7 (18) | 0 (0) | ||
| Total | 39 | 12 | ||
| CBR (%) | 16 (41.1) | 1 (8.3) | 0.04 | |
| PR + SD (%) | 16 (41.1) | 1 (8.3) | ||
| PD (%) | 15 (38) | 11 (92) | ||
| NA | 8 (21) | 0 (0) | ||
| Total | 39 | 12 | ||
Fisher’s exact test.
ORR: objective response rate, which includes confirmed responses only, RR: response rate, which includes confirmed or unconfirmed responses, CBR: clinical benefit rate which is defined as SD plus PR, NA: not available, PR: Partial Response, SD: Stable Disease, PD: Progressive Disease
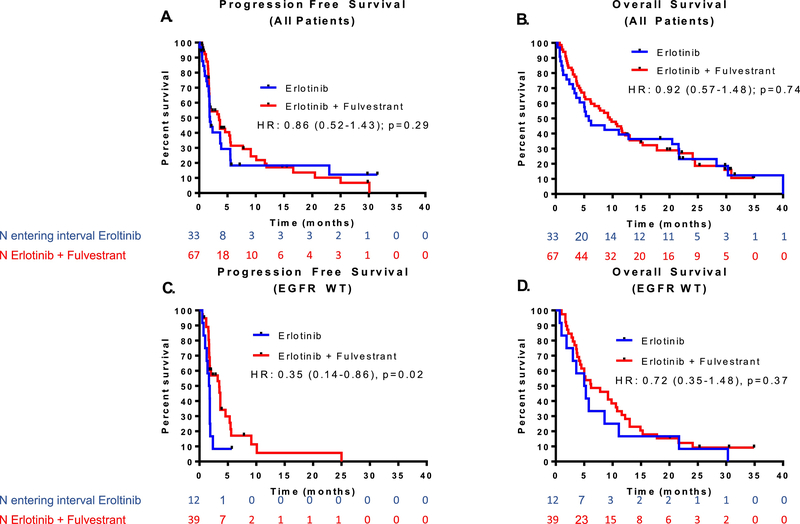
PFS was analyzed when 90 events had occurred. At the time of analysis, 82 deaths had occurred (54 patients receiving erlotinib plus fulvestrant and 28 patients receiving erlotinib alone). Overall, the median PFS was 3.5 months for erlotinib plus fulvestrant and 1.9 months for erlotinib alone [HR = 0.86, 95% CI (0.52–1.43), one-sided p=0.29] based on a log-rank test (Figure 2A). The median OS was 9.5 months for erlotinib plus fulvestrant versus 5.8 months for erlotinib alone [HR = 0.92, 95% CI, (0.57 to 1.48), two-sided p=0.74] based on a log-rank test (Figure 2B).
Figure 2.
Progression free survival (PFS) and overall survival (OS) data among all patients and subgroup analysis among EGFR wild type (WT) patients. A,B) PFS and OS for all patients, C,D) PFS and OS for EGFR WT patients only.
Within the EGFR WT subset, there was a significant PFS improvement with erlotinib plus fulvestrant. Median PFS was 3.5 and 1.7 months for erlotinib plus fulvestrant versus erlotinib alone [HR=0.35, 95% CI (0.14–0.86), p=0.02] (Figure 2C). A trend toward favorable OS with erlotinib plus fulvestrant was noted with a median OS of 6.2 months for erlotinib plus fulvestrant versus 5.2 months for erlotinib alone [HR=0.72, 95% CI (0.35–1.48), p=0.37] (Figure 2D). In order to determine if the significant PFS association with treatment in EGFR WT patients was potentially driven by gender, we also ran a Cox proportional hazards model for PFS with both treatment and gender and found that including gender did not substantially change the results. The treatment effect was still significant [HR=0.68, 95% CI (0.47–0.98), p=0.04] and effect for male [HR=1.38, 95% CI (0.72–2.62), p=0.33].
As anticipated, when comparing participants regardless of treatment arm, ORR, PFS, and OS were greatly superior in EGFR mutant patients when compared to EGFR WT. ORR regardless of treatment arm was 69% (11 of 17) versus 3.9% (2 of 51) in the EGFR mutant versus EGFR WT, respectively. Median PFS was 16.6 compared to 1.9 months [HR=0.25, 95% CI (0.13–0.47), p<0.01] (Supplemental Figure 1A), and median OS was 29.7 compared to 5.8 months [HR=0.28, 95% CI (0.16–0.50), p<0.01] (Supplemental Figure 1B). The number of EGFR mutant patients was too small to make between arm comparisons.
Analysis of Outcome by Staining for ER-𝛼, PgR and Blood Estrone and Estradiol
Fifty-six cases had tissue available to be tested for ER-α and PgR by immunochemistry, 2 of which were among the 6 patients who did not receive therapy. Among the remaining 54 cases, 48 had available clinical outcome data. No clear difference in ER-α or PgR staining by response amongst all cases (p=0.45), treatment arm (p=0.61), or EGFR WT patients in the combination treatment arm (p>0.99) were seen. However, we found that EGFR WT patients were more likely to be hormone receptor-positive (either ERα- and/or PgR-positive) compared to EGFR mutant patients (50% versus 9.1%, respectively) (p=0.03) (Table 3).
Table 3.
ER-α and PgR tissue immunohistochemistry analysis
| Hormone Receptor Positive1 |
Hormone Receptor Negative2 |
*P Value | ||
|---|---|---|---|---|
| All Patients | ||||
| ORR (%) | 2 (10) | 7 (21) | 0.45 | |
| PR (%) | 2 (10) | 7 (21) | ||
| SD (%) | 7 (35) | 10 (29) | ||
| PD (%) | 9 (45) | 13 (38) | ||
| NA (%) | 2 (10) | 4 (12) | ||
| Total | 20 | 34 | ||
| EGFR Hormone Receptor Status | ||||
| EGFR Mutant (%) | 1 (9.1) | 10 (90.9) | 0.03 | |
| EGFR WT (%) | 18 (50) | 18 (50) | ||
| Erlotinib and Fulvestrant Arm | ||||
| ORR (%) | 1(7) | 4 (20) | 0.61 | |
| PR (%) | 1 (7) | 4 (20) | ||
| SD (%) | 6 (40) | 7 (35) | ||
| PD (%) | 6 (40) | 8 (40) | ||
| NA (%) | 2 (13) | 1 (5) | ||
| Total | 15 | 20 | ||
| Erlotinib and Fulvestrant Arm with EGFR WT | ||||
| ORR (%) | 1 (7) | 1 (7) | >0.99 | |
| PR (%) | 1 (7) | 1 (7) | ||
| SD (%) | 6 (40) | 5 (36) | ||
| PD (%) | 6 (40) | 7 (50) | ||
| NA (%) | 2 (13) | 1 (7) | ||
| Total | 15 | 14 | ||
Fisher’s exact test.
Either ER-α or PgR status is positive
Both ER-α and PgR status is negative
ORR: objective response rate, which includes confirmed responses only, NA: not available, PR Partial Response, SD: Stable Disease, PD: Progressive Disease, ER: Estrogen Receptor; PgR: Progesterone Receptor
Estradiol and estrone levels were evaluated by response (Supplemental Figure 2) and treatment arm (Supplemental Figure 3). Evaluation of baseline levels was highly confounded by gender and menopausal status, making interpretation of this data difficult. Among 61 evaluable patients in which both cycle 1 and cycle 2 levels were obtained, the median difference in estradiol levels between cycles 1 and 2 (cycle 1 estradiol level minus cycle 2 estradiol level) was 2.3 pg/mL and 1.8 pg/mL among patients with progressive disease versus those with partial response and stable disease, respectively (p=0.20) by Wilcoxan rank sum, (Supplemental Figure 2C). Among 66 evaluable patients in which both cycle 1 and cycle 2 levels were obtained, the median difference in estradiol levels between cycles 1 and 2 (cycle 1 estradiol level minus cycle 2 estradiol level) was 1.7 versus 3.2 pg/mL among patients in the erlotinib and fulvestrant combination versus erlotinib alone, respectively (p=0.03) by Wilcoxan rank sum, (Supplemental Figure 3C).
3.3. Safety and Tolerability
The most common treatment-related AEs (all grades) in the evaluable population (n=100) were rash (65.7% versus 45.5%), diarrhea (43.3% versus 39.4%), fatigue (32.8% versus 18.2%), and anorexia (25.4% versus 27.3%) for erlotinib plus fulvestrant versus erlotinib alone, respectively (Table 4). There was no statistical difference in AEs between the treatment arms. The most common grade 3–4 AEs included diarrhea, rash, anorexia, and/or fatigue. The majority of the events were of grade 1 or 2 severity and manageable with standard supportive care. Two deaths occurred during the study, one due to respiratory failure in the erlotinib plus fulvestrant group and one due to cardiac-ischemia/infarction in the erlotinib alone group. Neither event was considered to be drug-related.
Table 4.
Most common adverse events and treatment-related events
| Erlotinib and Fulvestrant (n=67) |
Erlotinib Alone (n=33) |
*P value (All Grades) |
|||
|---|---|---|---|---|---|
| Any Adverse Event | All Grades (%) | Grade 3/4 (%) | All Grades (%) | Grade 3/4 (%) | |
| Anorexia | 22 (33) | 2 (3) | 10 (30) | 1 (3) | 0.799 |
| Diarrhea | 30 (45) | 3 (5) | 16 (49) | 2 (6) | 0.726 |
| Dyspnea | 22 (33) | 5 (8) | 6 (18) | 3 (9) | 0.125 |
| Fatigue | 29 (43) | 3 (5) | 12 (36) | 1 (3) | 0.508 |
| Infection | 16 (24) | 6 (9) | 5 (15) | 1 (3) | 0.314 |
| Nausea | 13 (19) | 2 (3) | 9 (27) | 0 (0) | 0.372 |
| Pain | 38 (57) | 7 (10) | 15 (46) | 2 (6) | 0.289 |
| Rash | 44 (66) | 4 (6) | 15 (46) | 0 (0) | 0.053 |
|
Treatment Related Adverse Event |
All Grades (%) | Grade 3/4 (%) | All Grades (%) | Grade 3/4 (%) | |
| Anorexia | 17 (25) | 2 (3) | 9 (27) | 1 (3) | 0.839 |
| Diarrhea | 29 (43) | 3 (5) | 13 (39) | 2 (6) | 0.711 |
| Fatigue | 22 (33) | 3 (5) | 6 (18) | 0 (0) | 0.129 |
| Nausea | 10 (15) | 1 (2) | 8 (24) | 0 (0) | 0.254 |
| Pain | 15 (22) | 1 (2) | 9 (27) | 0 (0) | 0.591 |
| Rash | 44 (66) | 15 (46) | 15 (46) | 0 (0) | 0.053 |
Fisher’s exact test.
Most common adverse events were defined as those that occurred in at least 25% of patients
4. Discussion
This open-label, randomized phase II trial examined the efficacy and safety of combination erlotinib plus fulvestrant compared to erlotinib alone for treatment of advanced NSCLC. Although there was no improvement in PFS or OS with erlotinib plus fulvestrant among all patients, in subgroup analysis among EGFR WT patients, erlotinib plus fulvestrant demonstrated an improvement in PFS. We acknowledge the EGFR WT subgroup analysis was not a preplanned analysis, but do recognize that these results corroborate preclinical studies that estrogen and EGFR play a significant role in lung cancer pathogenesis. During the design and subsequent course of this study from 2006 to 2011, the use of tyrosine kinase inhibitors (TKI) in EGFR mutant patients was not yet elucidated, and clinical guidelines for the use of erlotinib in NSCLC evolved significantly over the course of a few years. Initially, an EGFR TKI was recommended after two lines of prior therapy, and later treatment guidelines shifted to include a recommendation for an EGFR TKI after one or more lines of therapy. Among metastatic EGFR mutant patients, erlotinib therapy was recommended for front-line therapy [42, 43]. After these updated guidelines were implemented, there was a notable increase in the enrollment of EGFR mutant patients in this trial [3, 4, 44], making the prospective threshold for ORR difficult to achieve in order to demonstrate significant benefit with erlotinib plus fulvestrant.
In this trial, the OS and PFS for patients with an EGFR mutation were similar between the two treatment arms. The balance of EGFR mutant patients in the combination and erlotinib arms were similar, 15 and 21%, respectively. Importantly, among the unplanned EGFR WT subgroup analysis, we note that prior investigations have reported on differences in steroid hormone receptor expression in EGFR mutant as compared to EGFR WT tumors in the clinic [23, 45–48]. Our findings show that steroid hormone receptor expression is greater in EGFR WT tumors versus EGFR mutant tumors among patients enrolled on this trial. Consequently, the improved PFS and trend toward improved ORR and OS with the combination therapy among EGFR WT patients may be due in part to enhanced antiestrogen interactions with steroid hormone receptors enriched in the EGFR WT population. This finding is consistent with preclinical data demonstrating that estrogen stimulates specific gene expression, induces proliferation and growth, and stops apoptosis in NSCLC [14–29]. However, since this study began patient recruitment, the benefit of EGFR TKIs in terms of PFS and OS in the treatment of EGFR WT NSCLCs has come under question, culminating in the recent withdrawal of approval from the US Food and Drug Administration (FDA) in this setting [42, 49]. The authors acknowledge that the improved PFS among EGFR WT patients is important as proof of principle and would not advise taking this concept forward among EGFR WT patients without further substantial evidence to support it. Furthermore, because this is a subgroup analysis the sample size is small (n=51) with 39 and 12 patients in the combination versus erlotinib alone group, respectively. As stated by the US FDA in 2016, treatment with erlotinib should include only those patients with activating EGFR mutations [50]. Most clinical trials report that no significant improvements in PFS or OS occur in patients with EGFR WT tumors treated with erlotinib as compared to placebo controls [10]. This would indicate that the increase in PFS in EGFR WT patients treated with fulvestrant plus erlotinib in the current trial may have had clinical benefit due primarily to the addition of the antiestrogen fulvestrant. This outcome may be due primarily to an antiestrogen effect or to synergy between fulvestrant and erlotinib in EGFR WT patients. Thus, although this study endured slow patient accrual during a time of tremendous change in clinical practice, the improvement in PFS in patients with EGFR WT NSCLC treated with erlotinib and fulvestrant as compared to erlotinib alone is notable. Although not statistically significant, treatment-related rash and fatigue were higher in the combination arm, which are known side effects of fulvestrant therapy.
Apart from the differences in steroid hormone expression between wild type and mutant EGFR tumors noted above, additional studies of ER-α and PgR tissue expression and blood estradiol and estrone levels in this trial did not prove informative with regard to the selection of patients more likely to respond to hormonal interventions. As in studies in steroid hormone receptor-positive breast cancer, either ER-α and/or PgR positivity was considered hormone- receptor-positive, as ER-α if activated can induce PgR expression, thereby serving as a further biomarker of ER-α activity [51, 52]. Further studies are required to assess ER-α expression as an alternate biomarker using either validated ER-β antibodies as available and/or baseline transcript levels in NSCLC specimens, as ER-β is reported to be the predominant ER form in lung development and in malignant lung cells [14–26]. A comprehensive analysis including ER-β is planned pending the availability of a reliable and validated ER-β antibody for immunohistochemistry studies.
Further development of fulvestrant, other antiestrogens, or aromatase inhibitors in EGFR WT NSCLC should likely pursue alternate strategies rather than in combination with EGFR inhibitors. Additional trials of fulvestrant in postmenopausal women with advanced NSCLC are currently recruiting patients (NCT01556191). Alternative approaches would also include aromatase inhibitors such as exemestane alone (NCT02666105) or in combination with chemotherapy (carboplatin and pemetrexed) (NCT01664754) [53]. In addition, since the initiation of this study, immune checkpoint inhibitors have become an important intervention in lung cancer care [54, 55] and the possibility that hormonal therapies could complement immunotherapy is a potential focus of future research [56].
Supplementary Material
Highlights:
Phase II trial assessed antitumor efficacy of fulvestrant plus erlotinib in NSCLC
ORR was 16.4% for combination arm versus 12.1% for erlotinib alone arm
PFS and OS numerically favored the combination treatment arm versus erlotinib arm
In EGFR WT patients, but not EGFR mutant patients, fulvestrant plus erlotinib increased PFS
Dual therapy benefit in EGFR WT patients may be due in part to greater HR positivity
Acknowledgements:
This work was supported by the National Institutes of Health grant numbers K23CA149079, P50 CA090440, SPORE CDA FDP-NIH CA090388, NIH CTSI UL1 TR 000124, One Ball Matt Memorial Golf Tournament, V Foundation for Cancer Research, UCLA Jonsson Comprehensive Cancer Center, Wolfen Family Lung Cancer Research Program, Stiles Program in Oncology, Hickey Foundation, National Lung Cancer Partnership, Genentech, AstraZeneca, and F. Hoffmann-La Roche Ltd. This project used the University of Pittsburgh Small Molecule Biomarker Core for analysis of estradiol and estrone.
Funding: This work was supported by the National Institutes of Health grant numbers K23CA149079, P50 CA090440, SPORE CDA FDP-NIH CA090388, NIH CTSI UL1 TR 000124, One Ball Matt Memorial Golf Tournament, V Foundation for Cancer Research, UCLA Jonsson Comprehensive Cancer Center, Wolfen Family Lung Cancer Research Program, Stiles Program in Oncology, Hickey Foundation, National Lung Cancer Partnership, Genentech, AstraZeneca, and F. Hoffmann-La Roche Ltd.
Footnotes
Conflict of Interest Disclosure Statement: Dr. Edward B. Garon receives research funding from AstraZeneca and Genentech. All funding and acknowledgements are stated in the manuscript. The Corresponding Author, Dr. Edward Garon, is the sole contact for the Editorial process. He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary data for this manuscript can be found online.
References:
- 1.Jemal A, et al. , Cancer statistics, 2003. CA Cancer J Clin, 2003. 53(1): p. 5–26. [DOI] [PubMed] [Google Scholar]
- 2.Sharma SV, et al. , Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer, 2007. 7(3): p. 169–81. [DOI] [PubMed] [Google Scholar]
- 3.Lynch TJ, et al. , Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med, 2004. 350(21): p. 2129–39. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, et al. , Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med, 2005. 353(2): p. 123–32. [DOI] [PubMed] [Google Scholar]
- 5.Yang JC, et al. , Afatinib versus cisplatin-based chemotherapy for EGFR mutationpositive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol, 2015. 16(2): p. 141–51. [DOI] [PubMed] [Google Scholar]
- 6.Rosell R, et al. , Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol, 2012. 13(3): p. 239–46. [DOI] [PubMed] [Google Scholar]
- 7.Seto T, et al. , Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol, 2014. 15(11): p. 1236–44. [DOI] [PubMed] [Google Scholar]
- 8.Spigel DR, et al. , Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: Results from the pivotal phase III randomized, multicenter, placebocontrolled METLung (OAM4971g) global trial. Journal of Clinical Oncology, 2014. 32(15). [DOI] [PubMed] [Google Scholar]
- 9.Laurie SA and Goss GD, Role of epidermal growth factor receptor inhibitors in epidermal growth factor receptor wild-type non-small-cell lung cancer. J Clin Oncol, 2013. 31(8): p. 1061–9. [DOI] [PubMed] [Google Scholar]
- 10.Cicenas S, et al. , Maintenance erlotinib versus erlotinib at disease progression in patients with advanced non-small-cell lung cancer who have not progressed following platinum- based chemotherapy (IUNO study). Lung Cancer, 2016. 102: p. 30–37. [DOI] [PubMed] [Google Scholar]
- 11.The Coronary Drug Project. Findings leading to discontinuation of the 2.5-mg day estrogen group. The coronary Drug Project Research Group. JAMA, 1973. 226(6): p. 652–7. [PubMed] [Google Scholar]
- 12.Ganti AK, et al. , Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol, 2006. 24(1): p. 59–63. [DOI] [PubMed] [Google Scholar]
- 13.Chlebowski RT, et al. , Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet, 2009. 374(9697): p. 1243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stabile LP, et al. , Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res, 2002. 62(7): p. 2141–50. [PubMed] [Google Scholar]
- 15.Pietras RJ, et al. , Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids, 2005. 70(5–7): p. 372–81. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg OK, et al. , Aromatase inhibitors in human lung cancer therapy. Cancer Res, 2005. 65(24): p. 11287–91. [DOI] [PubMed] [Google Scholar]
- 17.Niikawa H, et al. , Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res, 2008. 14(14): p. 4417–26. [DOI] [PubMed] [Google Scholar]
- 18.Kerr A 2nd, Eliason JF, and Wittliff JL, Steroid receptor and growth factor receptor expression in human nonsmall cell lung cancers using cells procured by laser-capture microdissection. Adv Exp Med Biol, 2008. 617: p. 377–84. [DOI] [PubMed] [Google Scholar]
- 19.Jeong Y, et al. , Nuclear receptor expression defines a set of prognostic biomarkers for lung cancer. PLoS Med, 2010. 7(12): p. e1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marquez-Garban DC, et al. , Targeting aromatase and estrogen signaling in human nonsmall cell lung cancer. Ann N Y Acad Sci, 2009. 1155: p. 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olivo-Marston SE, et al. , Serum estrogen and tumor-positive estrogen receptor-alpha are strong prognostic classifiers of non-small-cell lung cancer survival in both men and women. Carcinogenesis, 2010. 31(10): p. 1778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garon EB, et al. , Antiestrogen fulvestrant enhances the antiproliferative effects of epidermal growth factor receptor inhibitors in human non-small-cell lung cancer. J Thorac Oncol, 2013. 8(3): p. 270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raso MG, et al. , Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res, 2009. 15(17): p. 5359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miki Y, et al. , Suppression of estrogen actions in human lung cancer. Mol Cell Endocrinol, 2011. 340(2): p. 168–74. [DOI] [PubMed] [Google Scholar]
- 25.Siegfried JM and Stabile LP, Estrongenic steroid hormones in lung cancer. Semin Oncol, 2014. 41(1): p. 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazmi N, et al. , The role of estrogen, progesterone and aromatase in human non-small- cell lung cancer. Lung Cancer Manag, 2012. 1(4): p. 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang H, et al. , Fulvestrant-mediated inhibition of estrogen receptor signaling slows lung cancer progression. Oncol Res, 2014. 22(1): p. 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okubo S, et al. , Additive antitumour effect of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib (Iressa, ZD1839) and the antioestrogen fulvestrant (Faslodex, ICI 182,780) in breast cancer cells. Br J Cancer, 2004. 90(1): p. 236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stabile LP and Siegfried JM, Estrogen receptor pathways in lung cancer. Curr Oncol Rep, 2004. 6(4): p. 259–67. [DOI] [PubMed] [Google Scholar]
- 30.Pietras RJ and Marquez-Garban DC, Membrane-associated estrogen receptor signaling pathways in human cancers. Clin Cancer Res, 2007. 13(16): p. 4672–6. [DOI] [PubMed] [Google Scholar]
- 31.Pietras RJ, Nemere I, and Szego CM, Steroid hormone receptors in target cell membranes. Endocrine, 2001. 14(3): p. 417–27. [DOI] [PubMed] [Google Scholar]
- 32.Marquez DC, et al. , Epidermal growth factor receptor and tyrosine phosphorylation of estrogen receptor. Endocrine, 2001. 16(2): p. 73–81. [DOI] [PubMed] [Google Scholar]
- 33.Traynor AM, et al. , Pilot study of gefitinib and fulvestrant in the treatment of postmenopausal women with advanced non-small cell lung cancer. Lung Cancer, 2009. 64(1): p. 51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Press M, et al. , Comparison of different antibodies for detection of progesterone receptor in breast cancer. Steroids, 2002. 67(9): p. 799–813. [DOI] [PubMed] [Google Scholar]
- 35.Finn RS, et al. , Estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2 (HER2), and epidermal growth factor receptor expression and benefit from lapatinib in a randomized trial of paclitaxel with lapatinib or placebo as first-line treatment in HER2-negative or unknown metastatic breast cancer. J Clin Oncol, 2009. 27(24): p. 3908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finn RS, et al. , Quantitative ER and PgR assessment as predictors of benefit from lapatinib in postmenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res, 2014. 20(3): p. 736–43. [DOI] [PubMed] [Google Scholar]
- 37.Hammond ME, et al. , American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol, 2010. 28(16): p. 2784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammond ME, et al. , American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med, 2010. 134(6): p. 907–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson RE, et al. , Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem, 2004. 50(2): p. 373–84. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, et al. , Analysis of estrogens and androgens in postmenopausal serum and plasma by liquid chromatography-mass spectrometry. Steroids, 2015. 99(Pt A): p. 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawaguchi T, et al. , Prospective Analysis of Oncogenic Driver Mutations and Environmental Factors: Japan Molecular Epidemiology for Lung Cancer Study. J Clin Oncol, 2016. 34(19): p. 2247–57. [DOI] [PubMed] [Google Scholar]
- 42.Fukuoka M, et al. , Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol, 2011. 29(21): p. 2866–74. [DOI] [PubMed] [Google Scholar]
- 43.Herbst RS, et al. , TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol, 2005. 23(25): p. 5892–9. [DOI] [PubMed] [Google Scholar]
- 44.Mok TS, et al. , Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med, 2009. 361(10): p. 947–57. [DOI] [PubMed] [Google Scholar]
- 45.Deng F, et al. , Correlation between epidermal growth factor receptor mutations and the expression of estrogen receptor-beta in advanced non-small cell lung cancer. Oncol Lett, 2017. 13(4): p. 2359–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He Q, et al. , Correlation between epidermal growth factor receptor mutations and nuclear expression of female hormone receptors in non-small cell lung cancer: a meta-analysis. J Thorac Dis, 2015. 7(9): p. 1588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu K, et al. , Membrane-bound estrogen receptor-alpha expression and epidermal growth factor receptor mutation are associated with a poor prognosis in lung adenocarcinoma patients. World J Surg Oncol, 2012. 10: p. 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willmore-Payne C, Holden JA, and Layfield LJ, Detection of epidermal growth factor receptor and human epidermal growth factor receptor 2 activating mutations in lung adenocarcinoma by high-resolution melting amplicon analysis: correlation with gene copy number, protein expression, and hormone receptor expression. Hum Pathol, 2006. 37(6): p. 755–63. [DOI] [PubMed] [Google Scholar]
- 49.Garassino MC, et al. , Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol, 2013. 14(10): p. 981–8. [DOI] [PubMed] [Google Scholar]
- 50.httD://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.Ddf 2016.
- 51.Flototto T, et al. , Molecular mechanism of estrogen receptor (ER)alpha-specific, estradiol-dependent expression of the progesterone receptor (PR) B-isoform. J Steroid Biochem Mol Biol, 2004. 88(2): p. 131–42. [DOI] [PubMed] [Google Scholar]
- 52.Allred DC, et al. , NCCN Task Force Report: Estrogen Receptor and Progesterone Receptor Testing in Breast Cancer by Immunohistochemistry. J Natl Compr Canc Netw, 2009. 7 Suppl 6: p. S1–S21; quiz S22–3. [DOI] [PubMed] [Google Scholar]
- 53.Young PA M-GD, Strunck J, AH Li, Adame C, Wells C, Spiegel M, Carroll J, Tucker DA, Famenini S, Callahan RD, Wong DJ, Goldman JW, Pietras RJ, Garon EB. IASLC Santa Monica Conference 2016 Phase I Dose Escalation Study of Carboplatin, Pemetrexed and Exemestane in Postmenopausal Women with Metastatic Non-Squamous, Non-Small Cell Lung Cancer (NSCLC). . IASLC 16th Annual Targeted Therapies of Lung Cancer Meeting, 2016. [Google Scholar]
- 54.Garon EB, et al. , Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med, 2015. 372(21): p. 2018–28. [DOI] [PubMed] [Google Scholar]
- 55.Borghaei H, et al. , Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small- Cell Lung Cancer. N Engl J Med, 2015. 373(17): p. 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamilton DH, et al. , Targeting estrogen receptor signaling with fulvestrant enhances immune and chemotherapy-mediated cytotoxicity of human lung cancer. Clin Cancer Res, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.