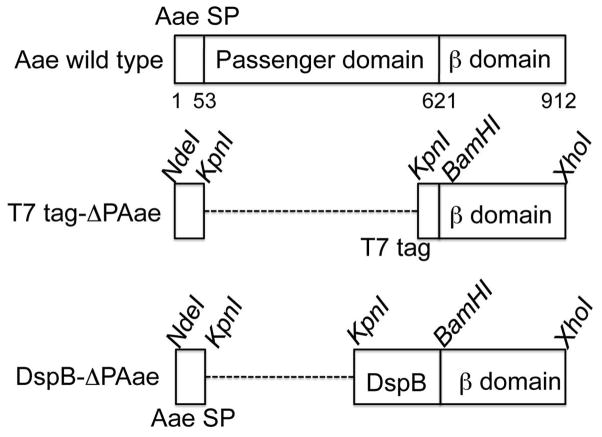
Figure 1.
Domain organization of the autotransporter Aae from A. actinomycetemcomitans. Unlike other autotransporters, Aae is lacking a protease like domain at the N-terminus of the extruded passenger domain. The numbers refer to the aa position of the start and end of the signal sequence and the β-domain, respectively. In the middle, the T7-tag containing hybrid with the Aae signal sequence (SP) and associated restriction enzymes sites are shown. When fused together, the hybrid protein will lack the passenger domain of Aae completely and in its place will have the T7 tag. At the bottom, the DspB/Aae hybrid with associated restriction sites are shown. This hybrid will lack the Aae passenger domain but will have DspB as the new passenger domain. In the truncated mutants, the signal sequence and the passenger domains are linked and the dashed line is used to keep the β-domains aligned in the figure.