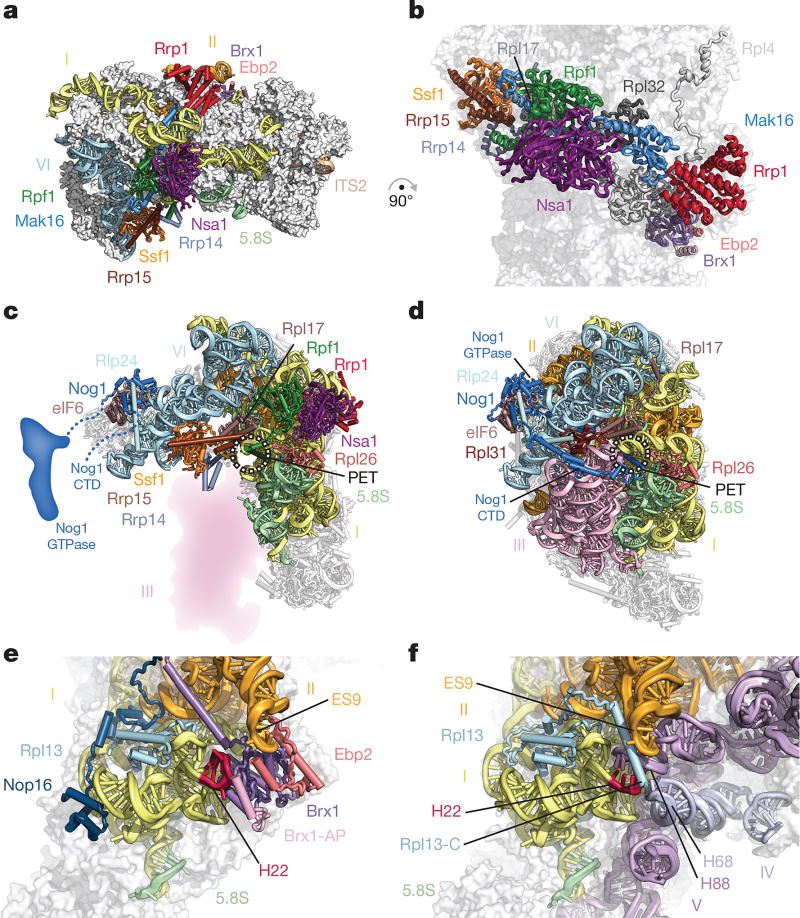
Figure 2. A ring of nucleolar assembly factors prevents premature folding of the 25S rRNA.
a, b, Assembly factors chaperone areas of domains I, II and VI and interact with ribosomal proteins. c, d, Assembly factors prevent the formation of the polypeptide exit tunnel (PET). c, In state 2, Ssf1–Rrp15 blocks the binding of Rpl31. Nog1 is largely unstructured. d, In the Nog2 particle (PDB 3JCT), the PET is formed, probed by Nog1 and supported by Rpl31. e, f, Brx1–Ebp2 and an associated peptide (Brx1-AP) remodel domain I (helix 22) to prevent binding of domain V (helix 88) and domain IV (helix 68) (e); these interactions are present in the Nog2 particle (f).