Abstract
A new fullerene (BB4-PPBA) functionalized with a tertiary amine and carboxylic acid was prepared and compared with BB4 (cationic quaternary group) for antimicrobial photodynamic inactivation (aPDI). BB4 was highly active against Gram-positive methicillin resistant Staphylococcus aureus (MRSA) and BB4-PPBA was moderately active when activated by blue light. Neither compound showed much activity against Gram-negative Escherichia coli or fungus Candida albicans. Therefore, we examined potentiation by addition of potassium iodide. Both compounds were highly potentiated by KI (1–6 extra logs of killing). BB4-PPBA was potentiated more than BB4 against MRSA and E. coli, while for C. albicans the reverse was the case. Addition of azide potentiated aPDI mediated by BB4 against MRSA, but abolished the potentiation caused by KI with both compounds. The killing ability after light decayed after 24 h in the case of BB4, implying a contribution from hypoiodite as well as free iodine. Tyrosine was readily iodinated with BB4-PPBA plus KI, but less so with BB4. We conclude that the photochemical mechanisms of these two fullerenes are different. BB4-PPBA is more Type 2 (singlet oxygen) while BB4 is more Type 1 (electron transfer). There is also a possibility of direct bacterial killing by electron transfer, but this will require more study to prove.
Keywords: Antimicrobial photodynamic inactivation, Functionalized fullerenes, Potassium iodide potentiation, Singlet oxygen, Photoinduced electron transfer, Photochemical mechanism
1. Introduction
The relentless advance of multi-antibiotic resistance amongst pathogenic bacteria and fungi [1] has led to fears of “untreatable infections” emerging [2]. These dire predictions have motivated the investigation of antimicrobial photodynamic inactivation (aPDI) [3] as a new alternative approach. Killing of microbial cells by aPDI is independent of antibiotic resistance status, and is thought unlikely to induce resistance itself [4]. aPDI relies on excitation of photosensitizing dyes called photosensitizers (PS) by visible light that can undergo an intersystem crossing to produce a long-lived triplet state [5]. The triplet state can react by two different photochemical pathways [6]. Type II involves energy transfer to surrounding (triplet) oxygen to produce the reactive oxygen species (ROS) singlet oxygen (1O2). Type I involves an electron transfer that can produce superoxide, hydrogen peroxide and hydroxyl radicals (HO’). The ROS produced by both these pathways can damage biomolecules (lipids, proteins and nucleic acids) leading to death of microbial cells. aPDI can be used as a therapeutic approach for infectious disease by designing PS that selectively bind to microbial cells and not to host mammalian cells, and by using a short drug-light interval. When used against infections, the PS is topically delivered to the infected area, rather than injected intravenously as would be the case in photodynamic therapy (PDT) for cancer [7]. It has been found that in general PS that possesses a cationic charge function well as antimicrobial compounds [8].
In recent years we have published several papers demonstrating that a range of different PS can have their photoantimicrobial activity dramatically potentiated by addition of a range of different inorganic salts [9]. The PS have included such structures as porphyrins [10, 11], phenothiazinium salts [12], Rose Bengal [13], titanium dioxide [14] and fullerenes [15]. These salts have included potassium iodide [12], sodium azide [16], potassium thiocyanate [17], potassium selenocyanate [18] and potassium bromide [19]. Potassium iodide is perhaps the most useful of these salts, considering its non-toxicity and accepted medical applications. The mechanism of action appears to be twofold. Firstly, there is the oxidation of iodide anion to produce molecular iodine, which is present as I3- in the presence of excess iodide. Hypoiodite (HOI) may also be produced by oxidation of iodide. Both free iodine and hypoiodite are well known antimicrobial agents. Secondly there is the possibility of short-lived reactive iodine species (for instance iodine radicals (I• or I2•-) can be produced during the illumination period. These two possibilities can be distinguished by comparing the killing using the “in” format experiment where all the components (PS, KI, cells) are present during the light delivery, with the killing seen in the “after” format experiment where the PS + KI mixture is illuminated and then the cells are added after the light is switched off.
Fullerenes are closed cage carbon compounds entirely composed of sp2 hybridized carbon atoms, originally named after Buckminster Fuller who designed the geodesic dome in architecture [20]. These compounds (including the commonest member C60) have a high molar absorbance coefficient for short wavelength visible light. They have a high quantum yield of triplet state formation and the ability to carry out photoinduced electron transfer reactions [21]. The photochemical mechanism of photoexcited fullerenes is highly dependent on their microenvironment. When illuminated in organic solvents they produce singlet oxygen in an almost quantitative manner, while in aqueous environments, they undergo Type I reactions especially in the presence of reducing agents [22]. Pristine fullerenes are highly insoluble and prone to agglomeration, so it is necessary to functionalize them to allow them to be used as PS in biological environments [23]. We have previously described the use of many different functionalized fullerenes as antimicrobial PS [24–27], including BB4 (!V,!V-dimethyfulleropyridinium bromide) [28] and several other analogous cationic fullerenes [29].
The present study aimed to compare BB4 with a newly prepared fullerene derivative BB4-PPBA for applications in aPDI, and proceeded to explore potentiation with the addition of KI salt and to investigate the operative photochemical mechanisms.
2. Methods
2.1. Chemistry
Synthesis of BB4 was carried out as previously described [28]. The synthesis of BB4-PPBA was carried out using methods previously described in the literature [30] with some modifications. We performed the synthesis, modifying the method to use dichlorobenzene as reaction solvent. In our current Prato reactions, we use dichlorobenzene to increase the solubility of C60 in order to reduce the amount of solvent that is used per reaction. Using our modified Prato protocol, we were able to synthesize BB4-PPBA, (25%, structure elucidated via MALDI and NMR).
BB4-PPBA:1H NMR (500 MHz, THF-d8 (ref @ 3.58 ppm) S (ppm): 11.5 br s (1H, COOH), 8.09 d (2H, J = 8.5 Hz, Ar-H), 7.97 m (2H, Ar-H), 5.12 s (1H, pyrrolidine 3 °C-H), 5.08 d (1H, 2JHH = 9.5 Hz, pyrrolidine 2 °C-H), 4.34 d (1H, 2JHh = 9.5 Hz, pyrrolidine 2 °C-H), 2.81 s (3H, N-CH3). MALDI-MS (negative mode, THAP): m/z 896.01 [ΜΗ]-, calculated 896.07 for C7oH10N02-.
2.2. Materials
Potassium iodide, sodium azide and IV-acetyl tyrosine ethyl ester were purchased from Sigma-Aldrich (St. Louis, MO). Stock solutions of fullerenes were freshly prepared each day by diluting a dimethylace-tamide (5 mM) solution into distilled H20 (dH20), and salt (iodide or azide) solution at 5 M. Phosphate-buffered saline (PBS) for microbial cell suspension and serial dilutions, brain-heart infusion broth (BHI), and agar for bacterial growth were purchased from Fisher Scientific (Waltham, MA). Starch indicator was purchased from RICCA Chemical Company (Arlington, TX). Amplex red hydrogen peroxide/peroxidase assay kit, singlet oxygen sensor green (SOSG) and hydroxyphenyl-fluorescein (HPF) were purchased from Invitrogen (Carlsbad, CA).
2.3. Cells and Culture Conditions
In this study we used methicillin-resistant Staphylococcus aureus (MRSA) USA 300 as Gram-positive bacteria; Escherichia coli K-12 (ATCC 33780) as Gram-negative bacteria and a luciferase-expressing Candida albicans strain (CEC 749) as fungal yeast. A colony of bacteria or fungal yeast was suspended in 20 mL of brain heart infusion (BHI) broth for bacteria or yeast extract-peptone-dextrose (YPD) broth for C. albicans and grown overnight in a shaker incubator (New Brunswick Scientific, Edison, NJ) at 120 rpm under aerobic conditions at 37 °C for bacteria, at 30 °C for C. albicans. For bacteria, an aliquot of 1 mL from an overnight bacterial suspension was refreshed in fresh BHI for 2–3 h at 37 °C to mid-log phase. Cell concentration was estimated by measuring the optical density (OD) at 600 nm (OD of 0.6 = 10s cells/mL). The C. albicans cell number was assessed with a hemocytometer and was generally between 107 and 10s cells/mL. The suspensions were centrifuged (5 min, 4000 rpm) and the pellet was suspended in sterile phosphate-buffered saline (PBS) at concentrations of 107−8CFU/mL for phototoxicity experiments.
2.4. In Vitro aPDI
A cell suspension consisting of 10s cells/mL for bacteria and 107 cells/mL for C. albicans in PBS was incubated with different concentration of BB4 and BB4-PPBA (up to 100 μM for E. coli and C. albicans) for 30 min at room temperature in the dark, then irradiated with different fluences of blue light 415 nm. The light source we used was an Omnilux Clear-U light-emitting diode (LED) array (Photo Therapeutics, Inc., Carlsbad, CA) that emitted blue light at a center wavelength of 415 nm. Care was taken to ensure that the contents of the wells were mixed thoroughly before sampling, as bacteria can settle at the bottom. The aliquots were serially diluted tenfold in PBS to give dilutions of 10−1 to 10−5 times in addition to the original concentration and 10 μL aliquots of each of the dilutions were streaked horizontally on square BHI agar plates for bacteria or YPD agar plates for Candida. Plates were streaked in triplicate and incubated for 12–18 h at 37 °C (bacteria) or for 24–36 h at 30 °C (Candida) in the dark to allow colony formation. Each experiment was performed at least three times.
Controls groups included cells that were not treated with fullerene or light, and cells treated with light but not with fullerene. Survival fractions (SF) were routinely expressed as ratios of CFU of microbial cells treated with light and fullerene or treated with fullerene in the dark, to CFU of microbes treated with neither.
2.5. Potentiation of aPDI by Addition of Potassium Iodide(KI)
To investigate the dependency of bacterial inactivation on KI concentration, we performed the same experiment using different concentrations of fullerene (BB4 = 100 nM and BB4-PPBA = 1 μM) for MRSA, 10 μM for E. coli and 100 μM for C. albicans and 10 J/cm2 of blue light and added a range of KI concentrations between 0 and 400 mM just before delivering the light. Then exposed to 10 J/cm2 blue light, the aliquots were serially diluted as before. Each experiment was performed at least three times.
2.6. Mechanisms of aPDI Potentiation
In order to test if the addition of KI can increase the fullerene-mediated PDI bacteria inactivation, we performed experiments in threedifferent formats. For the “In” format experiment, suspensions of bacteria (10s cells/mL) were incubated in the dark at room temperature for 30 min with BB4 (100 nM for MRSA and 10 μM for E. coil) or BB4-PPBA (1 μM for MRSA and 10 μM for E. coli) and added a range of KI concentrations between 0 and 400 mM and/or added sodium azide (NaN3, 50 mM) in pH 7.4 PBS. After exposure to 10 J/cm2 blue light, the aliquots were serially diluted as before. For the “Spin” format experiment, centrifugation (5 min, 4000 rpm) of 1 mL aliquots was used to remove the excess of BB4 or BB4-PPBA that was not taken up by the microbial cells, and after that we added 400 mM KI and/or 50 mM NaN3. Then the suspensions were irradiated with 10 J/cm2 blue light, and the aliquots were serially diluted as before. For the “After” format experiment, BB4 (100 nM, 10 μM or 100 μM) or BB4-PPBA (1 μM, 10 μM or 100 μM) with or without 400 mM KI and/or 50 mM NaN3 in pH 7.4 PBS were exposed to 10 J/cm2 blue light (cell free), then was added MRSA cells to the light-activated 100 nM BB4 or 1 μM BB4-PPBA group, while E. coli cells were added to the light activated 10 μM BB4 or BB4-PPBA group and the suspensions were thoroughly mixed. After 1 h incubation, the aliquots were serially diluted as before. Each experiment was performed at least three times.
2.7. Addition of Bacteria After Light Activation of Fullerene
A variation of the “after” format experiment was designed to investigate the microbial killing effect of the solution produced after light activation of fullerene plus KI, and test how long the photoproducts retained their antibacterial activity. 10 μM BB4 or BB4-PPBA and 400 mM KI were irradiated with 20 J/cm2 of 415 nm blue light, after the light was switched off, we removed aliquots at different times (0, 1 h, 2 h, 24 h) and added these aliquots to MRSA or E. coli cell suspension (10s/mL). After lh incubation period, the aliquots were serially diluted as before. Each experiment was performed at least three times.
2.8. Fluorescent Probe Assay for Generation of Specific ROS
Cell-free fluorescent-probe experiments utilized 96-well plates with a transparent bottom and black walls. 10 μM BB4 or BB4-PPBA with or without 400 mM KI in PBS, and singlet oxygen sensor green (SOSG) or hydroxyphenyl-fluorescein (HPF) (Molecular Probes, Invitrogen, USA) was added to each well at a final concentration oflO μM. Each experimental group contained four wells. All groups were illuminated simultaneously with 415 nm blue light, and light was delivered in sequential doses starting at 0 and going up to 70 J/cm2. A microplate spectrophotometer (Spectra Max M5 plate reader; Molecular Devices, Sunnyvale, CA) was used for acquisition of fluorescence signals in the “slow kinetic” mode. The fluorescence excitation was 505 nm and emission was 525 nm for SOSG, for HPF the excitation was 490 nm and emission was 515 nm. Each time after an incremental fluence was delivered, the fluorescence was measured.
2.9. Amplex Red Assay for Hydrogen Peroxide
Amplex red hydrogen peroxide/peroxidase assay kit was used to detect the production of H202 from fullerene + KI mediated PDT. The colorless probe Amplex red (10-acetyl-3, 7-dihydroxy-phenoxazine) reacts with H2O2 in the presence of peroxidase and forms resorufin (7-hydroxy-3H-phenoxazin-3-one). The detection process after 10 μM fullerene + 400 mMKI mediated PDT was according to manufacturer’s instructions. The reaction volume contained 10 μM BB4 or BB4-PPBA with added 400 mM KI were illuminated with increasing fluence of 415 nm blue light and aliquots withdrawn and added to 50 μM Amplex Red reagent and O.lU/mL horseradish peroxidase (HRP) in Krebs-Ringer phosphate (145 mM NaCl, 5.7 mM Na3P04, 4.86 mM KC1, 0.54 mM CaCl2, 1.22 mM MgS04, 5.5 mM glucose, pH 7.35). After 30 min incubation, a fluorescence microplate reader (excitation 530 nm and emission 590 nm) was used to measure incremental fluorescence after each fluence of blue light was delivered. Controls were [1] fullerene + light, [2] KI + light, and [3] Amplex red reagent alone. Each experiment was performed at least three times.
2.10. Iodine Starch Test
10 μM BB4 or BB4-PPBA with or without 400 mM KI in PBS were illuminated with increasing fluences of 415 nm blue light, and light was delivered in sequential doses from 0 to 100 J/cm2. Aliquots (50 μL) were withdrawn after each fluence, and added to starch indicator solution (50 μL). A microplate reader (absorbance 610 nm) was used to measure incremental absorbance after each fluence of 415 nm light was delivered. Controls were [1] fullerene + light, [2] KI + light, and [3] PBS alone. Each experiment was performed at least three times.
2.11. Iodination of N-acetyl Tyrosine Ethyl Ester
Sample solutions (total volume 500 μL) contained 100 μm bb4 or BB4-PPBA, 400mMKI and 10 mM N-acetyl-L-tyrosine ethyl ester in PB buffer (pH 7.4, containing 10% methanol) were irradiated by 415 nm blue light with magnetic stirring. An aliquot of solution (100 μL) was removed at different light dose (30 J/cm2, 60 J/cm2, 120 J/cm2) and centrifuged at 4000 rpm. It was necessary to use relatively large fluences of light in order to get enough product to allow measurement of the peak area. The supernatants were collected for the LC-MS analysis. The LC-MS analyses were performed on an Agilent 1260 LC system equipped with a triple-quad mass spectrometer. The LC conditions were: column: C18, 2.1 × 50 mm, 1.8 pm; elution gradient: solution A = acetonitrile, solution B = 10 mM ammonium acetate in water, 2% – > 100% of A over 6 min with a flow rate of 0.2 mL/min; ionization mode: negative; injection volume: 5 μL. The mass of the molecular ions of N-acetyl-3-iodo-L-tyrosine ethyl ester was 377.
3. Results
3.1. Synthesis of Compounds
The structures and the absorption spectra of the two fullerenes and the overlay of the blue light excitation source are shown in Fig. 1.
Fig. 1.

Structure and absorption spectra of fullerenes. (A) BB4; (B) BB4-PPBA; (C) Absorption spectra and overlapping emission spectrum of blue light. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Comparison of aPDI Studies
Fig. 2 shows the killing curves of the three different types of microbial cells mediated by both fullerenes excited by blue light. BB4 was highly active against MRSA, (Fig. 2A) producing eradication with only 1 μM and 20 J/cm2. BB4-PPBA was moderately active against MRSA (Fig. 2D) with eradication obtained at 5 μM and 20 J/cm2. BB4 showed only very low activity against E. coli (Fig. 2B) with the very high concentration of 100 μM only producing 1 log of killing after 20 J/cm2, while BB4-PPBA demonstrated no killing of E. coli at all even with these high doses (Fig. 2E). Against C. albicans BB4 produced only 2 logs of killing at the high concentration of 100 μM (Fig. 2C). Interestingly BB4-PPBA showed somewhat higher against C. albicans activity compared to BB4, producing over 4 logs of killing at 100 μM (Fig. 2F).
Fig. 2.

Comparison of aPDI activities of BB4 and BB4-PPBA. Cells (108/mL for bacteria and 107/mL for fungi) were incubated for 30 min with increasing concentrations of BB4 or BB4-PPBA and exposed to 10 or 20 J/cm2 of 415 nm light. (A, B, C) BB4; (D, E, F) BB4-PPBA. (A, D) MRSA; (B, E) E. coli; (C, F) C. albicans. The asterisk (*) in the plots indicates eradication (zero CFU).
3.3. Potentiation by Addition of KI
Because the killing of Gram-negative bacteria and fungi by these photoactivated fullerenes was overall disappointing, we asked whether the addition of KI could produce a significant potentiation, as we had previously demonstrated using PS with other chemical structures that were highly active against Gram-positive bacteria, but were inactive against other microbial cell types [10, 11, 13]. We used a series of increasing concentrations of KI (up to 400 mM) because we had previously shown that relatively high KI concentrations were necessary to achieve maximum potentiation. We attempted to adjust the basic aPDI parameters (PS concentration and fluence) to produce about one log of killing without any KI, in order to observe high degree of potentiation. This meant that for MRSA we used different concentrations of fullerene (BB4 = 100 nM and BB4-PPBA = 1 μM) because BB4 alone was substantially more active than BB4-PPBA alone. For the other cells where neither compound had much activity alone, we used the same concentration for both compounds (10 μM for E. coli and 100 μM for C. albicans). Fig. 3A shows that for MRSA, the potentiation with BB4-PPBA was higher (eradication with 200 mM KI) while with BB4, 400 mM KI was needed to achieve 5 logs of killing. Interestingly in the case of BB4, intermediate concentrations of KI (100 and 200 μM) gave protection from killing rather than potentiation. Potentiation with BB4-PPBA was also higher with E. coli (Fig. 3B) with BB4-PPBA producing eradication with 400 mM KI, while only 2 logs of killing was found with BB4 plus 400 mM KI. The situation with C. albicans was the opposite (Fig. 3C) with BB4 giving more potentiation than BB4-PPBA, but no eradication was obtained. Considering that we were already using a very high PS concentration (100 μM) we did not continue to include C. albicans in our studies.
Fig. 3.

Potentiation of aPDI mediated by BB4 and BB4-PPBA by addition of KI. Cells (108/mL for bacteria and 107/mL for fungi) were incubated for 30 min with 100 nM of BB4 for MRSA, or 1 μM of BB4-PPBA for MRSA, or with 10 μM of BB4 or BB4-PPBA for E. coli and C. albicans, different concentrations (up to 400 mM) of KI were added and then exposed to 10 J/cm2 of 415 nm light or kept in the dark as controls. (A) MRSA; (B) E. coli; (C) C. albicans. The asterisk (*) in the plots indicates eradication (zero CFU).
3.4. Mechanisms of a PDI Potentiation
In order to gain information on the mechanisms involved in the aPDI activity and KI potentiation, we carried out the following experiments. We used the same concentrations of fullerenes as in the previous section, and compared the killing using the “in” format with that obtained using the “after” format. We also used the “spin format”. Here the cells are incubated with the PS, then centrifuged and resuspended before the KI is added and the light is delivered. The goal is to try to distinguish between short lived reactive species that will only kill bacteria when they are produced very close to the cells, as the fullerenes remaining after centrifugation are bound to the cell surface. We also added sodium azide (50 mM) because with the Type 2 photochemical mechanism, azide is thought to quench singlet oxygen and reduce killing, while with the Type 1 mechanism, azide may not quench the killing [31] and may even “paradoxically” potentiate the killing [16, 32].
Fig. 4 shows the results. With BB4 (100 nM) against MRSA (Fig. 4A) eradication was achieved by addition of 400 mM KI using both “in” and “spin” formats, but no killing at all was seen in the “after” format. When azide was added to aPDI alone there was potentiation in the “in” format with > 2 logs killing with azide as opposed to < 1 log with no azide. Slight potentiation was seen in the “spin” format. When azide was added to the aPDI + KI mixture, the killing seen with aPDI + KI was almost abolished in the “in” and “spin” formats. When BB4-PPBA (1 μM) was tested against MRSA (Fig. 4B) again the addition of 400 mM KI led to eradication in both the “in” format and the “spin” format. There was only a little (< 1 log) killing in the “after” format. Addition of azide did not produce significant potentiation of aPDI alone, while addition of azide to the aPDI + KI, markedly (but not completely) quenched the killing seen in the “in” and “spin” formats. When BB4 (10 μM) was tested against E. coli (Fig. 4C) eradication was observed in all three formats “in”, “after”, and “spin”. No significant killing was seen with azide + aPDI, and addition of azide completely quenched all the killing seen in all three formats. With BB4-PPBA (10 μM) against E. coli (Fig. 4D) eradication was seen with “in” and “after” formats but with the “spin” format there was 4 logs of killing (but not eradication). The difference between the two fullerenes with respect to the “spin” format is explained by the fact that the binding of BB4-PPBA to E. coli is weaker than that of BB4. There was no killing seen with azide plus aPDI, and addition of azide to aPDI + KI completely abolished the killing in all three formats.
Fig. 4.

Comparison of “in”, “after” and “spin” formats for fullerene-mediated aPDI with addition of KI and/or azide. Bacterial cells (108/mL) were incubated for 30 min with lOOnM of BB4 for MRSA, or 1 μM of BB4-PPBA for MRSA, or with 10 μM of BB4 or BB4-PPBA for E. coli and then centrifuged (“spin”) or not (“in”) followed by addition of 400 mM KI and/or 50 mM NaN3. For “after” solutions of fullerene and salt(s) were irradiated and then bacterial cells added and incubated for 1 h. In every case the light dose was 10 J/cm2 of 415 nm light. (A) BB4 & MRSA; (B) BB4-PPBA & MRSA; (C) E. coli & BB4; (D) E. coli & BB4-PPBA. The asterisk (*) in the plots indicates eradication (zero CFU).
3.5. Stability of the Antibacterial Substance Produced in the “After” Format
We had previously shown that both molecular iodine (I2/I3 ) and hypoiodite (HOI) could be produced by interaction of the ROS produced during aPDI with iodide anion. Both iodine product species are antibacterial, but the difference is that I3” is stable, while HOI slowly degrades over a period of hours. Hence by adding the cells to the irradiated mixture of the fullerene and KI after different lengths of time have elapsed, information on the balance of I3” and HOI may be obtained. It was necessary to increase the fullerene concentration up to 10 μM and the light up to 20 J/cm2 in order to achieve eradication in the “after” format. Fig. 5 shows the results. For MRSA (Fig. 5A) eradication was achieved at 0, 1, and 2h post light delivery. However, at 24 h post-light the killing had almost disappeared with BB4, while with BB4-PPBA the killing had reduced to > 2 logs. With E. coli (Fig. 4B) again eradication was obtained with both fullerenes at 0, 1, and 2h. At 24 h the killing with BB4 had disappeared while eradication was still achieved with BB4-PPBA.
Fig. 5.

Stability of the antibacterial species produced in the “after” format. Solutions of fullerene (10 μM) and KI (400 mM) were irradiated with 20 J/cm2 of 415 nm light and then bacterial cells (108/mL) were added at different times after the light was switched off, and incubated for 1 h. (A) BB4 or BB4-PPBA & MRSA; (B) BB4 or BB4-PPBA & E. coli. The asterisk (*) in the plots indicates eradication (zero CFU).
3.6. Chemical Assays in Solution
In an attempt to gain more information about the photochemical pathways operating with these photoexcited fullerenes, we used four chemical assays that can be used in solution to detect ROS and free iodine (Fig. 6). SOSG is a fluorescent probe that is considered to be specific for singlet oxygen [33], while HPF is considered to be specific for hydroxyl radicals [34], Amplex red is an assay for hydrogen peroxide in solution [34], and starch produces a well-known blue colored complex with triiodide. Fig. 6A shows the results with SOSG. It is known that blue light alone activates SOSG [35]. BB4-PPBA gave more activation than BB4 and its activation was substantially quenched by addition of KI. Fig. 6B shows the results with HPF. Activation was much higher with BB4-PPBA compared to BB4, and in both cases the activation was completely quenched by addition of KI. Fig. 6C shows the results with Amplex red. BB4-PPBA and BB4-PPBA plus KI produced similar amounts of H2C>2, while the addition of KI to BB4 produced several times more H202 compared to BB4 alone. Fig. 6D shows the production of blue starch color (indication of iodine production through formation of a starch-triiodide complex). No iodine was produced by blue light alone and KI, while BB4-PPBA plus KI produced 2–3 times more iodine compared to BB4 plus KI (Fig. 6D).
Fig. 6.

Mechanistic studies. In each case, wells containing fullerene (10 μM) with or without 400 mM KI were excited by increasing fluences of light. (A) Plates were read in fluorescence plate reader after each fluence of white light. SOSG (10 μM) ex/em 505/525 nm; (B) Fullerene excited by white light, HPF (10 μM) ex/em 490/515 nm. (C) Aliquots were withdrawn after fluences of 415 nm light and added to Amplex Red reagent. (D) Aliquots were withdrawn after fluences of 415 nm light and added to starch indicator reagent. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.7. Iodination of Tyrosine
The reactive species produced during the photochemical oxidation of KI may iodinate tyrosine. This is conveniently tested by using the ester-amide derivative of tyrosine, IV-acetyl-L-tyrosine ethyl ester which produces N-acetyl-3-iodo-tyrosine ethyl ester than can be quantitatively measured by LC-MS. Fig. 7 shows that only BB4-PPBA produced significant amounts of 3-iodotyrosine derivative while BB4 did not.
Fig. 7.
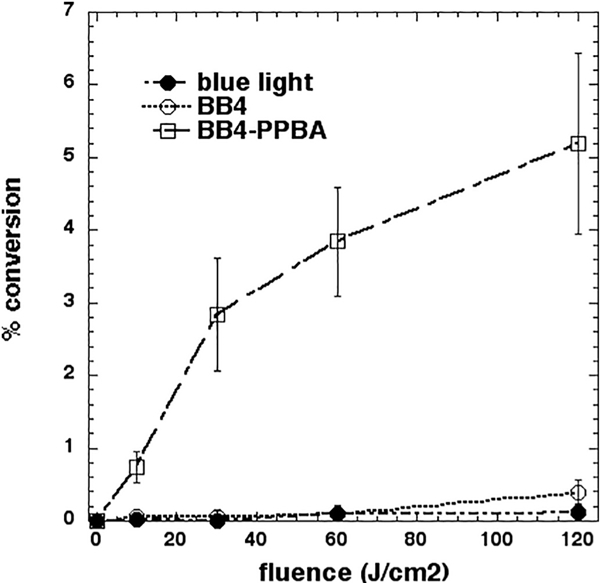
Iodination of N-acetyl tyrosine ethyl ester. Solutions of fullerene (100 μM), N-acetyl tyrosine ethyl ester (10 mM) and KI (400 mM) were exposed to 415 nm light. Aliquots were withdrawn and the product (N-acetyl-3-iodo-tyrosine ethyl ester) analyzed by LC-MS.
4. Discussion
Both BB4 and BB4-PPBA were highly active as PS against Grampositive bacteria such as MRSA. Concentrations as low as 100 nM could produce measurable killing of MRSA. BB4 was significantly more active (at least ten times) than BB4-PPBA, probably due to its better solubility and lesser tendency to agglomerate. In the case of Gram-negative E. coli neither compound had any appreciable activity, even though BB4 was marginally better than BB4-PPBA, which had no measurable impact. We previously compared the activity of BB4, with two analogous full-erenes, BB5 (two pyrrolidinium groups) and BB6 (three pyrrolidinium groups) as PS against Gram-positive and Gram-negative bacteria. We found that both BB5 and BB6 were much more active against E. coli and P. aeruginosa than BB4 [28].
The fungal yeast was intermediate in susceptibility between MRSA and E coli. Interestingly BB4-PPBA was significantly better than BB4 at killing Candida. The reasons for this are probably due to the more hydrophobic nature of BB4-PPBA compared to BB4. Hydrophobic compounds (for instance amphotericin B) are traditionally used to kill fungal cells such as Candida [36]. Since the overall results of aPDI with these two fullerenes could fairly be classed as disappointing, we asked whether the microbial killing could be potentiated by addition of the non-toxic salt KI. We had previously shown that the potentiating effect of KI is most pronounced against Gram-negative bacteria. When the PS does not have a pronounced cationic charge, it generally displays very poor activity (even zero activity) against Gram-negative species such as E. coli. We found that addition of 100 mM KI to 10 μM Photofrin excited by blue light could eradicate E. coli, as opposed to zero killing without KI [11]. Similar results (zero killing changing to 6 logs) were achieved with 10 μM Rose Bengal excited by green light [13] and the anionic porphyrin TPPS4 (10 μM) excited by blue light [10]. As might be expected in the present study, the KI potentiation was more pronounced with BB4-PPBA (because it had a much lower activity on its own) than it was with the more active BB4 where the already high activity was difficult to improve upon. The concentration of KI required to observe the full potentiation of aPDI (in most cases) was as high as 400 mM, while in the studies referred to above with different PS [10, 11, 13], a concentration of 100 mM KI was sufficient to produce eradication.
Because the two fullerenes had different activities on their own and had different propensities to be potentiated by addition of KI, we tested the hypothesis that these differences were due to the photochemical mechanism that predominates with each fullerene. Our tentative hypothesis was that BB4-PPBA was more Type II in nature because we believed that singlet oxygen was better at oxidizing iodide to the antibacterial free iodine than was Type I ROS. The production of free iodine leads to the formation of the triiodide anion (I3~) by combination with dissolved iodide, and triiodide production can be measured by the formation of a blue color with starch. The ability of a PS to produce the relatively stable triiodide species determines how much bacterial killing was observed in the “after” format. The fact that there was a lot more starch color indicative of free iodine production with BB4-PPBA confirmed this assumption. However, when we came to test the two fluorescent probes SOSG and HPF the results were not entirely what we expected. Although BB4-PPBA did produce more SOSG activation than BB4, neither fullerene produced much activation at all, compared to the values we have seen with other PS structures. For instance, both fullerenes (10 μM) produced less SOSG activation than Rose Bengal used at one hundredth of the concentration (100 nM) [13]. Likewise, we did not expect that BB4-PPBA would produce more activation of HPF than BB4, so that was also surprising. Moreover, the results with Amplex Red were also somewhat puzzling. BB4-PPBA produced more H202 than BB4, but the addition of KI produced a large increase in H202 in the case of BB4, but little increase in the case of BB4-PPBA. The results begin to suggest that bacterial photokilling mediated by fullerenes includes a contribution from a PDT mechanism we have considered in past studies, but have not discussed it much because it is rather difficult to prove. I am referring to the idea of direct photoinduced electron transfer from the excited state fullerene to the bacterial cells.
It is known that artificially increasing electron transport inside bacteria can potentiate killing, and indeed this concept is related to the theories from Prof J J Collins on how bactericidal antibiotics kill bacteria via generation of intracellular ROS [37, 38]. It is known that many bacteria (including Shewanella and Geobacter) can carry out what is known as “Extracellular Electron Transfer” [39, 40] where instead of the electrons that are produced in the electron transport chains being transferred to oxygen as the final electron acceptor, they are instead transferred to various minerals such as Fe(III) and Mn(IV). There has been recent research about the possibilities of constructing “microbial fuel cells” where bacteria convert organic compounds into electricity that could in principle be harvested for energy generation [41, 42]. What has not been much studied is the reverse of this process where electrons are pumped into bacteria [43]. There are several papers describing the killing of bacteria by low intensity direct electric currents where bacteria are growing as biofilms on electrodes made from graphite [44] or titanium [45]. Further investigation will be required to obtain hard evidence for this hypothetical mechanism in the case of photoactivated fullerenes.
Fullerenes are known to be excellent electron acceptors in photo-induced electron transfer processes [46]. Many of these processes take advantage of another molecule that acts simultaneously as a light-harvesting antenna and as an electron donor. These molecules can be phthalocyanines [47], porphyrins [48] or diphenylaminofluorene (DPAF) [49]. The goal of many of these studies is to prepare novel organic solar cells for electricity generation [50]. A dyad between a porphyrin and a Οβο fullerene designed to carry out photoinduced charge separation was able to carry out oxygen-independent cell photoinactivation [51], but no definitive studies were undertaken to establish if electron transfer into the cells was responsible.
The KI would be expected to serve as a good source of electrons in the photoinduced electron transfer process, and thus could explain the high degree of potentiation of killing observed by addition of KI (in the apparent absence of large amounts of ROS generation). Of course, the oxidized iodine species (iodine radicals or free iodine molecules) as discussed above are highly bactericidal. However, the lower iodine production, and significantly reduced tyrosine iodination in the case of BB4 suggests that the electron transfer route may not give the same iodide oxidation as the singlet oxygen route. The possibility of the formation of hypoiodite with BB4, and free iodine with BB4-PPBA, corroborates this difference. We did attempt to carry out some aPDI experiments using photoactivation of fullerenes in the presence of KI and in the absence of oxygen (data not shown), but the results showed a high degree of variability. Nevertheless, we believe that this possibility is worthy of further investigation. Even though it is highly likely that azide radicals were formed when azide ions were added to photoexcited BB4, it is difficult to be certain that these azide radicals are responsible for the potentiation in bacterial killing. It is equally plausible that the azide ions acted as electron donors to the fullerene thus increasing the “electron transfer killing”. Further experiments will be necessary to test this interesting (but as yet unproven) theory of “electron transfer killing”.
The theory about bacterial killing being due to direct electron transfer could explain another observation which has been somewhat puzzling to us. I refer to the large difference in aPDI effectiveness against Gram-positive bacteria between cationic fullerenes such as BB4 (this paper) and BB6 (Fig. 8A) [28] where a few hundred nanomolar concentration was sufficient, and the fullerenes we described in other publications [15, 25, 52–54] that had polycationic chains (5, 10 or 20 cationic charges (Fig. 8B). In those studies, much higher concentrations (at least 10 μM) of fullerene and more light was necessary to achieve killing. If the fullerene cage must reach close to the plasma membrane for the electron transfer to take place, then BB4 with its single charge close to the cage is well placed to achieve that goal with porous Grampositive bacterial cells. However, compounds like LC14, LC15, and LC16 [54] (Fig. 8C) each of which has two attached chains containing a total of ten cationic charges will be too bulky for the cage to make close contact with the plasma membrane. In the case of Gram-negative bacteria, BB4 is not sufficiently cationic to penetrate through the outer membrane barrier so therefore has little activity. Although the LC fullerenes do have 10 cationic charges and presumably can penetrate through the outer membrane of Gram-negative bacteria, the bulkiness of the molecule may still prevent the fullerene from making close enough contact with the plasma membrane. Of course, the above arguments would apply equally well to the generation of short lived ROS that cannot diffuse very far and needs to be generated in close contact with the target to be effective, but neither the present BB4 or the LC fullerenes generated the expected large amounts of ROS (although the LC fullerenes did generate more than BB4).
Fig. 8.

Chemical structures of other functionalized fullerenes mentioned in the discussion. BB6 [28], LC14 [52], LC16 [15].
In conclusion, although the aPDI killing of bacteria mediated by photoactivated fullerenes can be strongly potentiated by addition of KI, the lack of strong ROS production, and the need for high concentrations of KI, suggests the mechanism may be somewhat different from that proposed for the majority of PS with different structures. Further work is needed to test the interesting possibility of direct electron transfer to bacteria.
Acknowledgements
This work was supported by US NIH grants R01AI050875 and R21AI121700 (to MRH) and R43AI109938 to Lynntech, Inc. (SL). Weijun Xuan was supported by National Natural Science Foundation of China (81774374, 81373700, 81260552). Liyi Huang was supported by National Natural Science Foundation of China (81260239, 81472002), Guangxi Scientific and Technological Project (1355005–1-2), Guangxi Natural Science Foundation (2016GXNSFAA380312).
References
- [1].O’Neill J, Tackling a global health crisis:initial steps, The Review on Antimicrobial Resistance Chaired by Jim O’Neill, 2015. [Google Scholar]
- [2].Livermore DM, Has the era of untreatable infections arrived? J. Antimicrob. Chemother. 64 (Suppl. 1) (2009) i29–i36. [DOI] [PubMed] [Google Scholar]
- [3].Hamblin MR, Antimicrobial photodynamic inactivation: a bright new technique to kill resistant microbes, Curr. Opin. Microbiol 33 (2016) 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kashef N, Hamblin MR, Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updat. 31 (2017) 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wainwright M, Maisch T, Nonell S, Plaetzer K, Almeida A, Tegos GP, Hamblin MR, Photoantimicrobials-are we afraid of the light? Lancet Infect. Dis. 17 (2) (2017) e49–e55 February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baptista MS, Cadet J, Di Mascio P, Ghogare AA, Greer A, Hamblin MR, Lorente C, Nunez SC, Ribeiro MS, Thomas AH, Vignoni M, Yoshimura TM, Type I and type II photosensitized oxidation reactions: guidelines and mechanistic pathways, Photochem. Photobiol. 93 (2017) 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sharma SK, Mroz P, Dai T, Huang YY, St Denis TG, Hamblin MR, Photodynamic therapy for cancer and for infections: what is the difference? Isr. J. Chem. 52 (2012) 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Abrahamse H, Hamblin MR, New photosensitizers for photodynamic therapy, Biochem. J. 473 (2016) 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hamblin MR, Potentiation of antimicrobial photodynamic inactivation by in organic salts, Expert Rev. Anti-Infect. Ther (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang L, El-Hussein A, Xuan W, Hamblin MR, Potentiation by potassium iodide reveals that the anionic porphyrin TPPS4 is a surprisingly effective photosensitizer for antimicrobial photodynamic inactivation, J. Photochem. Photobiol. B 178 (2017) 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huang L, Szewczyk G, Sarna T, Hamblin MR, Potassium Iodide Potentiates Broad-Spectrum Antimicrobial Photodynamic Inactivation Using Photofrin, ACS Infect. Dis 3 (2017) 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vecchio D, Gupta A, Huang L, Landi G, Avci P, Rodas A, Hamblin MR, Bacterial photodynamic inactivation mediated by methylene blue and red light is enhanced by synergistic effect of potassium iodide, Antimicrob. Agents Chemother. 59 (2015) 5203–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wen X, Zhang X, Szewczyk G, El-Hussein A, Huang YY, Sarna T, Hamblin MR, Potassium iodide potentiates antimicrobial photodynamic inactivation mediated by Rose Bengal in in vitro and in vivo studies, Antimicrob. Agents Chemother. (2017) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huang YY, Choi H, Kushida Y, Bhayana B, Wang Y, Hamblin MR, Broad-spectrum antimicrobial effects of photocatalysis using titanium dioxide nano particles are strongly potentiated by addition of potassium iodide, Antimicrob. Agents Chemother. 60 (2016) 5445–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang Y, Dai T, Wang M, Vecchio D, Chiang LY, Hamblin MR, Potentiation of antimicrobial photodynamic inactivation mediated by a cationic fullerene by added iodide: in vitro and in vivo studies, Nanomedicine (Lond.) 10 (2015) 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang L, St Denis TG, Xuan Y, Huang YY, Tanaka M, Zadlo A, Sarna T, Hamblin MR, Paradoxical potentiation of methylene blue-mediated antimicrobial photodynamic inactivation by sodium azide: role of ambient oxygen and azide radicals, Free Radic. Biol. Med. 53 (2012) 2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].St Denis TG, Vecchio D, Zadlo A, Rineh A, Sadasivam M, Avci P, Huang L, Kozinska A, Chandran R, Sarna T, Hamblin MR, Thiocyanate potentiates anti microbial photodynamic therapy: in situ generation of the sulfur trioxide radical anion by singlet oxygen, Free Radic. Biol. Med. 65C (2013) 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huang L, Xuan W, Zadlo A, Kozinska A, Sarna T, Hamblin MR, Antimicrobial photodynamic inactivation is potentiated by addition of selenocyanate: possible involvement of selenocyanogen? J. Biophoton. 28 (2018) e201800029 February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wu X, Huang YY, Kushida Y, Bhayana B, Hamblin MR, Broad-spectrum anti microbial photocatalysis mediated by titanium dioxide and UVA is potentiated by addition of bromide ion via formation of hypobromite, Free Radic. Biol. Med. 95 (2016) 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stace AJ and O’Brien P, (2016). Fullerenes: past, present and future, celebrating the 30th anniversary of Buckminster Fullerene . Philos. Trans. A Math. Phys. Eng. Sci 374, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang YY, Sharma SK, Yin R, Agrawal T, Chiang LY, Hamblin MR, Functionalized fullerenes in photodynamic therapy, J. Biomed. Nanotechnol. 10 (2014) 1918–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y, Masumizu T, Nagano T, Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: 02-* versus 102, J. Am. Chem. Soc. 125 (2003) 12803–12809. [DOI] [PubMed] [Google Scholar]
- [23].Nakamura E, Isobe H, Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience, Acc. Chem. Res. 36 (2003) 807–815. [DOI] [PubMed] [Google Scholar]
- [24].Huang L, Terakawa M, Zhiyentayev T, Huang YY, Sawayama Y, Jahnke A,Tegos GP, Wharton T, Hamblin MR, Innovative cationic fullerenes as broad-spectrum light-activated antimicrobials, Nanomedicine 6 (2010) 442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huang L, Wang M, Dai T, Sperandio FF, Huang YY, Xuan Y, Chiang LY, Hamblin MR, Antimicrobial photodynamic therapy with decacationic mono adducts and bisadducts of [70]fullerene: in vitro and in vivo studies, Nanomedicine (Lond.) 9 (2014) 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang L, Wang M, Sharma SK, Sperandio FF, Maragani S, Nayka S, Chang J, Hamblin MR, Chiang LY, Decacationic [70]fullerene approach for efficient photokilling of infectious bacteria and cancer cells, ECS Trans 45 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mizuno K, Zhiyentayev T, Huang L, Khalil S, Nasim F, Tegos GP, Gali H, Jahnke A, Wharton T, Hamblin MR, Antimicrobial photodynamic therapy with functionalized fullerenes: quantitative structure-activity relationships, J. Nanomed. Nanotechnol 2 (2011) 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, Hamblin MR, Cationic fullerenes are effective and selective antimicrobial photo sensitizers, Chem. Biol. 12 (2005) 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mizuno K, Zhiyentayev T, Huang L, Khalil S, Nasim F, Tegos GP, Gali H, Jahnke A, Wharton T, Hamblin MR, Antimicrobial photodynamic therapy with functionalized fullerenes: quantitative structure-activity relationships, J. Nanomed. Nanotechnol 2 (2011) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Poddutoori PK, Sandanayaka ASD, Hasobe T, Ito O, van der Est A, Photoinduced charge separation in a ferrocene — aluminum(III) porphyr in—fullerene supramolecular triad, J. Phys. Chem. B 114 (2010) 14348–14357. [DOI] [PubMed] [Google Scholar]
- [31].Huang L, Xuan Y, Koide Y, Zhiyentayev T, Tanaka M, Hamblin MR, Type I and type II mechanisms of antimicrobial photodynamic therapy: an in vitro study on gram-negative and gram-positive bacteria, Lasers Surg. Med 44 (2012) 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kasimova KR, Sadasivam M, Landi G, Sarna T, Hamblin MR, Potentiation of photoinactivation of Gram-positive and Gram-negative bacteria mediated by six phenothiazinium dyes by addition of azide ion, Photochem. Photobiol. Sci. 13 (2014) 1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gollmer A, Arnbjerg J, Blaikie FH, Pedersen BW, Breitenbach T, Daasbjerg K, Glasius M, Ogilby PR, Singlet Oxygen Sensor Green(R): photochemical behavior in solution and in a mammalian cell, Photochem. Photobiol. 87 (2011) 671–679. [DOI] [PubMed] [Google Scholar]
- [34].Price M, Reiners JJ, Santiago AM, Kessel D, Monitoring singlet oxygen and hydroxyl radical formation with fluorescent probes during photodynamic therapy, Photochem. Photobiol. 85 (2009) 1177–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ragas X, Jimenez-Banzo A, Sanchez-Garcia D, Batllori X, Nonell S, Singlet oxygen photosensitisation by the fluorescent probe Singlet Oxygen Sensor Green, Chem. Commun. (Camb.) (2009) 2920–2922. [DOI] [PubMed] [Google Scholar]
- [36].Gidaro MC, Alcaro S, Seed D, Rivanera D, Mollica A, Agamennone M, Giampietro L, Carradori S, Identification of new anti-Candida compounds by ligand-based pharmacophore virtual screening, J. Enzym. Inhib. Med. Chem. 31 (2016) 1703–1706. [DOI] [PubMed] [Google Scholar]
- [37].Lobritz MA, Belenky P, Porter CB, Gutierrez A, Yang JH, Schwarz EG, Dwyer DJ, Khalil AS, Collins JJ, Antibiotic efficacy is linked to bacterial cellular respiration, Proc. Natl. Acad. Sci. U. S. A. 112 (2015) 8173–8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ, A common mechanism of cellular death induced by bactericidal antibiotics, Cell 130 (2007) 797–810. [DOI] [PubMed] [Google Scholar]
- [39].Kato S, Biotechnological aspects of microbial extracellular electron transfer, Microb. Environ. 30 (2015) 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shi L, Dong H, Reguera G, Beyenal H, Lu A, Liu J, Yu HQ, Fredrickson JK, Extracellular electron transfer mechanisms between microorganisms and minerals, Nat. Rev. Microbiol. 14 (2016) 651–662. [DOI] [PubMed] [Google Scholar]
- [41].Lovley DR, Bug juice: harvesting electricity with microorganisms, Nat. Rev. Microbiol. 4 (2006) 497–508. [DOI] [PubMed] [Google Scholar]
- [42].Lovley DR, The microbe electric: conversion of organic matter to electricity, Curr. Opin. Biotechnol. 19 (2008) 564–571. [DOI] [PubMed] [Google Scholar]
- [43].Nealson KH, Rowe AR, Electromicrobiology: realities, grand challenges, goals and predictions, Microb. Biotechnol. 9 (2016) 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Niepa THR, Wang H, Gilbert JL, Ren D, Eradication of Pseudomonas aeruginosa cells by cathodic electrochemical currents delivered with graphite electrodes, Acta Biomater. 50 (2017) 344–352. [DOI] [PubMed] [Google Scholar]
- [45].Ercan B, Kummer KM, Tarquinio KM, Webster TJ, Decreased Staphylococcus aureus biofilm growth on anodized nanotubular titanium and the effect of electrical stimulation, Acta Biomater 7 (2011) 3003–3012. [DOI] [PubMed] [Google Scholar]
- [46].Ito O, Photosensitizing Electron Transfer Processes of Fullerenes, Carbon Nanotubes, and Carbon Nanohorns, Chem. Rec 17 (2017) 326–362. [DOI] [PubMed] [Google Scholar]
- [47].Ray A, Pal H, Bhattacharya S, Photophysical investigations on supramolecular fullerene/phthalocyanine charge transfer interactions in solution, Spectrochim . Acta A Mol. Biomol. Spectrosc. 117 (2014) 686–695. [DOI] [PubMed] [Google Scholar]
- [48].Ghosh BK, Bauri A, Bhattacharya S, Banerjee S, Photophysical investigations on effective and selective complexation of a designed monoporhyrin with C(6)(0) and C(7)(0) in solution, Spectrochim . Acta A Mol. Biomol. Spectrosc. 125 (2014) 90–98. [DOI] [PubMed] [Google Scholar]
- [49].El-Khouly ME, Padmawar P, Araki Y, Verma S, Chiang LY, Ito O, Photoinduced processes in a tricomponent molecule consisting of diphenylaminofluorene-dicya-noethylene-methano[60]fullerene, J. Phys. Chem. A 110 (2006) 884–891. [DOI] [PubMed] [Google Scholar]
- [50].Li Y, Molecular design of photovoltaic materials for polymer solar cells: toward suitable electronic energy levels and broad absorption, Acc. Chem. Res. 45 (2012) 723–733. [DOI] [PubMed] [Google Scholar]
- [51].Milanesio ME, Alvarez MG, Rivarola V, Silber JJ, Durantini EN, Porphyrin-fullerene C60 dyads with high ability to form photoinduced charge-separated state as novel sensitizers for photodynamic therapy, Photochem. Photobiol. 81 (2005) 891–897. [DOI] [PubMed] [Google Scholar]
- [52].Wang M, Huang L, Sharma SK, Jeon S, Thota S, Sperandio FF, Nayka S, Chang J, Hamblin MR, Chiang LY, Synthesis and photodynamic effect of new highly photostable decacationically armed [60]- and [70]fullerene decaiodide monoadducts to target pathogenic bacteria and cancer cells, J. Med. Chem. 55 (2012) 4274–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang M, Maragani S, Huang L, Jeon S, Canteenwala T, Hamblin MR, Chiang LY, Synthesis of decacationic [60]fullerene decaiodides giving photo induced production of superoxide radicals and effective PDT-mediation on anti microbial photoinactivation, Eur. J. Med. Chem. 63C (2013) 170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yin R, Wang M, Huang YY, Landi G, Vecchio D, Chiang LY, Hamblin MR, Antimicrobial photodynamic inactivation with decacationic functionalized fullerenes: oxygen independent photokilling in presence of azide and new mechanistic insights, Free Radic. Biol. Med 79 (2015) 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


