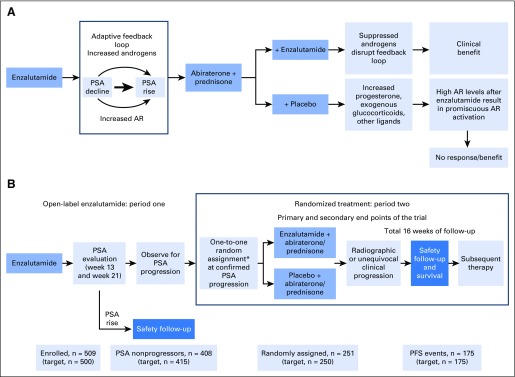
Fig 1.
(A) Scientific hypotheses underlying PLATO trial and (B) PLATO trial design. Actual patient numbers at each trial milestone are included on the bottom row of panel B, and target numbers are included in brackets. More details on period one patient disposition are provided in the Data Supplement. AR, androgen receptor; PFS, progression-free survival; PSA, prostate-specific antigen. (*) Random assignment was stratified by confirmed PSA response at week 13 in period one (≥ 0% to < 30% v ≥ 30%).