Abstract
Several aspects of stem cell life are governed by epigenetic variations, such as DNA methylation, histone modifications, and chromatin remodeling. Epigenetic events are also connected with the impairment of stem cell functions. For example, during senescence, there are significant changes in chromatin organization that alter transcription. The MECP2 protein can bind methylated cytosines and contribute to regulating gene expression at one of the highest hierarchical levels. Researchers are particularly interested in this protein, as up to 90% of Rett syndrome patients have an MECP2 gene mutation. Nevertheless, the role of MECP2 in this disease remains poorly understood. We used a mouse model of Rett syndrome to evaluate whether residual MECP2 activity in neural stem cells (NSCs) induced the senescence phenomena that could affect stem cell function. Our study clearly demonstrated that the reduced expression of MECP2 is connected with an increase in senescence, an impairment in proliferation capacity, and an accumulation of unrepaired DNA foci. Mecp2+/− NSCs did not cope with genotoxic stress in the same way as the control cells did. Indeed, after treatment with different DNA-damaging agents, the NSCs from mice with mutated Mecp2 accumulated more DNA damage foci (γ-H2AX+) and were more prone to cell death than the controls. Senescence in Mecp2+/− NSCs decreased the number of stem cells and progenitors and gave rise to a high percentage of cells that expressed neither stem/progenitor nor differentiation markers. These cells could be senescent and dysfunctional.
Rett syndrome: Mutation affects neural stem cells
In Rett syndrome, neural stem cells lose some of their “stem cell like” properties, impairing brain functions. Patients with this rare neurological condition, almost exclusively girls, show impaired movement and speech beginning at 6–18 months of age. Mutations in the MECP2 gene are known to be involved, but the specifics are poorly understood. Umberto Galderisi at Temple University in Philadelphia and co-workers in Italy used a mouse model to trace how MECP2 mutations affect neural stem cells. They found that the mutated cells lost key stem cell abilities, including the capacity to renew themselves by dividing, and the ability to differentiate, or turn into other cell types. The cells were also highly susceptible to DNA damage and unable to repair it. These results improve our understanding of Rett syndrome and may help develop new treatments.
Introduction
Changes in chromatin status occur through epigenetic events, such as DNA methylation, histone modifications, and chromatin remodeling. These phenomena modify the accessibility of genes to transcription factors and other modulators and are at the top of the hierarchy governing the regulation of gene expression.
Several aspects of stem cell life are governed by epigenetic variations that, for example, may repress genes associated with lineage specification and promote the expression of genes involved in maintaining stemness properties. Alternatively, epigenetic marks may induce a specific lineage commitment by the repression of genes associated with the differentiation to other lineages1.
In addition to having a physiological role in the control of stem cell biology, epigenetic events may be associated with impairment of their functions. Indeed, all cell types, including stem cells, undergo changes in chromatin organization and gene expression patterns as they senesce. This can be due to the derangement of chromatin modifiers induced by exogenous and endogenous stressors2.
Methylation of cytosines at CpG sites in mammalian cells is catalyzed by DNA methyltransferases and in general is associated with the compaction of chromatin and gene silencing. Proteins with a methyl-CpG binding domain can bind methylated DNA and further contribute to regulating gene expression3–5.
Among the various methylated DNA binding proteins, MECP2 (methyl-CpG binding protein 2) is of interest, as its mutation is associated with Rett syndrome, a severe neurological disease that almost exclusively affects girls6,7.
The MECP2 gene is ubiquitously expressed, and its mutations can impair the function of many other genes in neural cells and in other tissues and organs, such as bones and muscles. Specifically, mutations in MECP2 can alter the activity of stem cells. This, in turn, can have a profound effect on the life of an individual. In a previous finding, we evidenced that mesenchymal stem cells obtained from Rett patients are prone to senescence. These results were validated by in vitro studies on mesenchymal stem cells with a partial silencing of MECP2 expression. In this model, we demonstrated that senescence associated with a negligible expression of MECP2 occurred through canonical Rb- and p53-related pathways8–10.
We then decided to investigate the effect of impaired MECP2 function on the in vitro behavior of neural stem cells to evaluate if the senescence phenomena could affect these cells. This hypothesis took into consideration the fact that Rett syndrome patients present mainly neurological symptoms.
Materials and methods
Animals
Heterozygous B6.129P2(C)-Mecp2tm1Bird/J females and the corresponding wild-type animals were purchased from Jackson Laboratories. All animals were handled in compliance with protocols that were approved by the Animal Care and Use Committee of Campania University. Animals were acclimatized, quarantined, and then killed at 8–9 weeks of age to isolate and collect the brains.
Neurosphere cultures of neural stem cells (NSCs)
NSCs were grown as neurospheres11. Adult neurospheres were obtained from the brains of mouse strain B6.129P2(C)-Mecp2tm1.1Bird/J and the corresponding wild-type animals. Hippocampal and posterior subventricular zone brain areas were dissected. Tissue samples were minced and enzymatically digested. The cells were pooled, plated in Dulbecco's modified Eagle's medium (DMEM)/F-12 medium supplemented with epidermal growth factor (EGF) and fibroblast growth factor-2 (FGF-2), and incubated for 7–10 days to permit neurosphere formation. We verified that the cultures fulfilled the minimal proposed criteria to define NSCs: immunohistochemical detection was used to demonstrate that the NSCs expressed NESTIN and SOX2. By contrast, they did not express terminal differentiation markers12. To obtain reliable results, all of the experiments were performed on Mecp2+/− and wild-type cells on the same day as the in vitro cultivation.
Neurosphere differentiation and characterization
NSC differentiation was performed as we reported previously12. In brief, neurospheres were mechanically dissociated and plated in DMEM/F-12, 1 × B27 (all from ThermoFisher Italy) without EGF and FGF-2 at a density of 5 × 105 cells/ml onto poly-l-ornithine (Sigma-Aldrich Italy)-coated cell culture plates. Differentiated cells were identified by immunocytochemistry with a Neural Stem Cell Marker Characterization Kit (Millipore Italy). The kit contains primary antibodies against NESTIN, SOX2, MAP2, O1, and GFAP, which allow the identification of stem cells, early progenitors, and differentiated cells. The immunoreactions were performed according to the manufacturer’s instructions and our previous published protocols12. Cells expressing mutated or wild-type Mecp2 were identified by using the anti-MECP2 (clone Mec-168) primary antibody (Abcam, Cambridge, UK) according to the manufacturer’s protocol. The MECP2 protein was undetectable in cells expressing the mutated allele13.
Cell proliferation and cell cycle analysis
Cell growth was evaluated with the Quick Cell Proliferation Assay Kit II (Biovision, CA, USA). The assay determines the number of living cells by measuring the cleavage of the tetrazolium salt to formazan by cellular mitochondrial dehydrogenase at 440 nm in a multi-well spectrophotometer. For cell cycle analysis, the cells were fixed in 70% ethanol, followed by phosphate-buffered saline (PBS) washes, and then dissolved in a hypotonic buffer containing propidium iodide. The samples were acquired on a Guava EasyCyte flow cytometer (Merck Millipore, Italy) and analyzed with the manufacturer’s standard procedure using the EasyCyte software.
Apoptosis, necrosis, and senescence detection
Apoptosis was detected by means of a fluorescein-conjugated Annexin V Kit (Roche, Italy) by following the manufacturer’s instructions. Apoptotic cells were identified with a fluorescence microscope (Leica Italia, Italy). In every experiment, a minimum of 1000 cells were counted in different fields to calculate the percentage of apoptotic cells. Trypan blue staining was used to identify necrotic non-viable cells. In brief, trypsinized cell were incubated in a 0.2% Trypan blue solution for a few minutes, and the dead blue cells were identified with an inverted microscope. A minimum of 1000 cells were counted in different fields to calculate the percentage of dead cells.
Senescence was evaluated with a quantitative senescence-associated β-galactosidase assay. Essentially, 4-methylumbelliferyl-β-d-galactopyranoside (4-MUG) is a β-galactosidase substrate that does not emit fluorescence until cleaved by the enzyme to generate the fluorophore 4-methylumbelliferone. As already reported, we performed an assay on cell lysates to monitor the fluorophore production at an emission/excitation wavelength of 365/460 nm14.
Western blotting
Cells were lysed in a buffer containing 0.1% Triton for 30 min at 4 °C. Then, 40 μg of each lysate was electrophoresed in a polyacrylamide gel and electroblotted onto a nitrocellulose membrane. The primary antibodies LC3 (ab51520, Abcam), RB1 (C15, Santa Cruz Biotech), P21 (C19, Santa Cruz Biotech), P16 (ab54210, Abcam), and P53 (DOI1, Santa Cruz Biotech) were used according to the manufacturers’ instructions. The immunoreactions were performed with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotech), followed by an ECL Plus reagent (GE Healthcare).
Induction of DNA damage
For the hydrogen peroxide treatment, the NSCs were incubated for 1 h with 300 μM H2O2. The cells were then collected for data analysis 48 h later. For ultraviolet (UV) irradiation, the NSCs plated in cell dishes without lids were irradiated with UV light by exposure to a germicidal lamp (peak sensitivity approximately 365 nm) in a tissue culture hood (15 mJ/cm2). The cells were collected 48 h later. For doxorubicin treatment, the cells were incubated with 1 μM doxorubicin in complete culture medium for 24 h. The medium was then removed, and the cells were incubated for 24 h in fresh medium before further analysis.
Immunocytochemistry for the DNA repair pathway
The kinase ataxia–telangiectasia-mutated (ATM; ab36810, Abcam, UK), γ-H2AX (2577, Cell Signaling, MA, USA), RAD51 (ab88572, Abcam), and DNA-PK (sc390698, Santa Cruz Biotech) were detected according to the manufacturers’ protocols. Hoechst 33342 staining was performed, and the cells were observed with a fluorescence microscope (Leica Italia, Italy). The percentage of ATM-, γ-H2AX-, RAD51-, and DNA-PK-positive cells was calculated by counting a minimum of 500 cells in different microscope fields.
Statistical analysis
Statistically significant data were evaluated using a one-way analysis of variance analysis followed by Student’s t-test and Bonferroni’s test. A mixed-model variance analysis for data with continuous outcomes was used. All data were analyzed with the GraphPad Prism statistical software package version 5.01 (GraphPad, CA, USA).
Results
There are several mouse models for Rett syndrome15. Homozygote B6.129P2(C)-Mecp2tm1.1Bird/J females have been used to study the consequences related to the complete absence of MECP2 function. In the current study, we used heterozygote female mice of this strain to study the effects of partial MECP2 loss of function, as this condition may occur in girls with Rett syndrome. Indeed, in a previous in vitro investigation, we evidenced that even a mild reduction in MECP2 expression may impair stem cell functions9. We isolated NSCs from Mecp2+/− and wild-type animals and analyzed their biological properties.
NSCs from mutated animals showed growth reduction and senescence
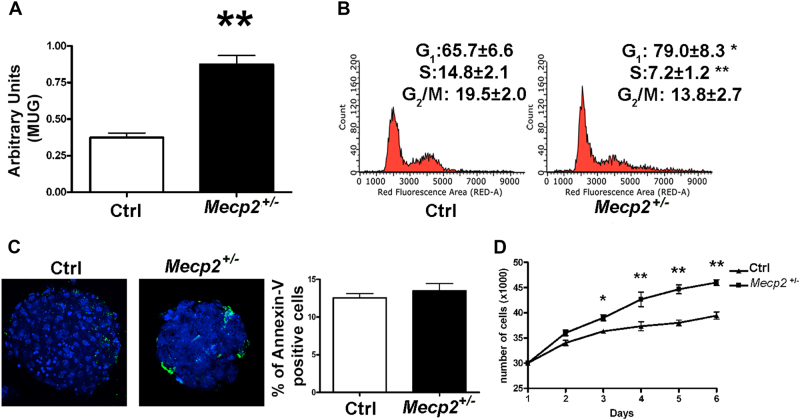
The MTT proliferation assay revealed that the NSCs from mutated animals grew at a significant slower rate compared with those from control animals (Fig. 1d). Consistent with this result, in the Mecp2+/− samples, we observed a reduction in the percentage of cells in S phase and a stall in G2/M (Fig. 1b).
Fig. 1. Cell growth, senescence, and apoptosis assays on Mecp2+/− and control NSCs.
a Senescence evaluation was performed with the MUG assay. The data are expressed as arbitrary units (±SD, n = 5 biological replicates, **p < 0.01). b Cell cycle analysis. The picture shows the cell cycle profiles of mutated and control NSCs (±SD, n = 5 biological replicates, *p < 0.05, **p < 0.01). c Annexin V assay to detect apoptotic cells. Representative photomicrographs of apoptotic cells stained with Annexin V (green). Nuclei were counterstained with Hoechst 33342 (blue). Graph shows mean expression values in Mecp2+/− and control samples (±SD, n = 5 biological replicates). d Cell proliferation was determined with a Quick Cell Proliferation Assay Kit II. Cells were seeded in 96-well culture plates. At 2, 3, 4, 5, and 8 days post plating, the cells were collected and counted (±SD, n = 5 biological replicates, *p < 0.05, **p < 0.01)
Growth reduction was not coupled with changes in the percentage of apoptotic cells but rather with a near doubling in the level of senescence (Fig. 1a, c).
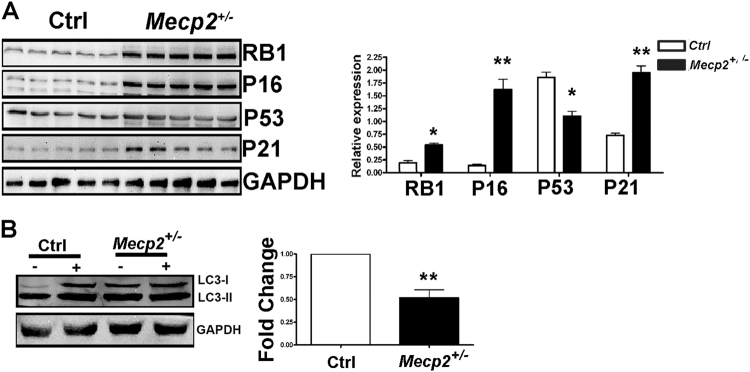
The reduction in cell growth and senescence onset were consistent with an increase in the levels of RB1, P21, and P16, which are part of the cell cycle arrest and senescence-associated pathways16. Of note, exit from the cell cycle and senescence were not related to P53, as we observed a decrease in this protein in the Mecp2+/− samples (Fig. 2).
Fig. 2. Western blot analysis of cell cycle-related pathways and autophagic flux.
a The picture shows the expression levels of RB1, P16, P53, and P21 in Mecp2+/− and control samples. For every protein, three biological replicates are shown. GAPDH was used as a loading control. The graph shows mean expression levels (±SD, n = 5 biological replicates, *p < 0.05, **p < 0.01). b Western blot detection of LC3-I and LC3-II in Mecp2+/− and control NSCs. The cells were grown as floating neurospheres and then harvested for western blot analysis. The detection of autophagic flux was performed by treating cultures with 100 nM of Bafilomycin A1, an inhibitor of lysosomal degradation, or with PBS. The LC3-I and II band intensities were normalized with GAPDH. The picture illustrates a representative western blot we analyzed to determine the autophagic flux. Autophagic flux (AF) for LC3-II was calculated as follows: Mecp2+/− NSC AF=(Mecp2+/− sample in Bafylomycin A1)−(Mecp2+/− sample in PBS), Control NSC AF=(Control sample in Bafylomycin A1)−(Control sample in PBS). The change in autophagic flux (ΔAF) between Mecp2+/− and the control NSCs was calculated as ΔAF=Mecp2+/− NSC AF− Control NSC AF. The graph shows AF changes in Mecp2+/− NSCs compared with the control cultures. The data in the control were set as equal to 1 (n = 5 biological replicates; **p < 0.01)
Mecp2+/− NSCs demonstrated an impairment in autophagic flux
Senescence may be associated with the modification of autophagy. We evaluated autophagic flux in Mecp2-mutated samples by determining the levels of two isoforms of the microtubule-associated protein 1 light chain 3 (LC3-I and LC3-II). During a normal autophagic process, LC3-I is converted into LC3-II, which is tightly bound to the autophagosome membrane17. We determined that the switch from LC3-I to LC3-II was impaired in NSCs obtained from mutated animals compared with the controls (Fig. 2).
NSCs from Mecp2-mutated mice are prone to DNA damage
The increase in senescence we detected in the Mecp2+/− NSCs prompted us to examine their degree of DNA damage by evaluating the expression level of γ-H2AX. Soon after a genotoxic stress that induces DNA damage is administered, the histone H2AX is phosphorylated to mark the damaged areas. This facilitates the recruitment and docking of DNA repair proteins. In the nucleus, the γ-H2AX foci disappear after a correct repair of DNA. The persistence of foci several hours or days after stress induction implies the presence of unrepaired DNA.
In the Mecp2+/− samples, we discovered an increase in the number of cells with evident signs of damaged DNA (Fig. 3a).
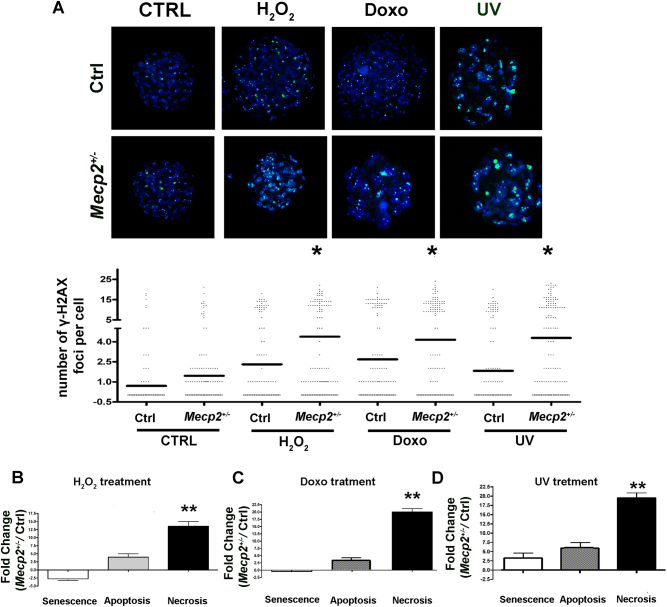
Fig. 3. DNA damage, senescence, apoptosis, and necrosis.
a Photos show the merging of cells stained with anti-H2AX (green) and DAPI (blue). Representative microscopic fields are depicted. The column scatter plot indicates the degree of H2AX phosphorylation that was determined by counting the number of γ-H2AX immunofluorescent foci per cell. The foci number was determined for 200 cells. Each dot represents a single cell. Horizontal bars indicate the mean value for each category (Mecp2+/− and control NSCs; n = 5 biological replicates; *p < 0.05). b, c, d The DNA damage levels following genotoxic treatments with hydrogen peroxide, doxorubicin, and UV irradiation, respectively. For every condition, the degree of DNA damage was determined by counting γ-H2AX foci. Changes in the level of senescence, apoptosis, and necrosis in Mepc2+/− NSCs were compared with the control cultures. The data are expressed in fold change (n = 5 biological replicates; *p < 0.05, **p < 0.01)
These data prompted us to hypothesize that a reduced MECP2 expression may render these cells more sensitive to DNA damage. We then treated NSCs with DNA-damaging agents (H2O2, UV radiation, and doxorubicin) to test our hypothesis. After each genotoxic treatment, we compared the level of senescence, apoptosis, and necrosis in Mecp2+/− and control NSCs. We also evaluated the number of unrepaired DNA foci by γ-H2AX staining.
Following treatment with hydrogen peroxide, NSCs from mutated animals showed a higher degree of apoptosis and necrosis than the control samples. This was associated with a significant increase in the mean number of H2AX foci per cell (Fig. 3b).
Treatment with doxorubicin and UV produced the same outcomes. Mecp2+/− NSCs entered necrosis much more frequently than the control cells, while apoptosis was reduced, and senescence remained unchanged (Fig. 3c, d). Moreover, in this case, we observed an increase in the percentage of cells with unrepaired or misrepaired DNA (Fig. 3c, d).
The NSC differentiation process is impaired in the Mecp2+/− samples
Senescence may affect cell commitment and differentiation; therefore, we analyzed NSC differentiation by identifying the percentage of stem cells, precursors, and mature cells in the samples from mutated and wild-type animals (Fig. 4).
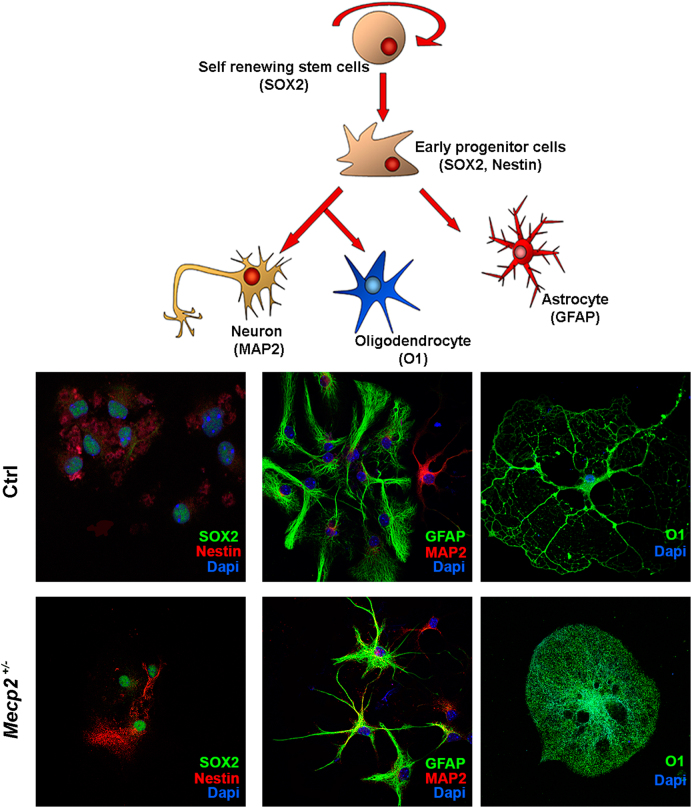
Fig. 4. NSC differentiation.
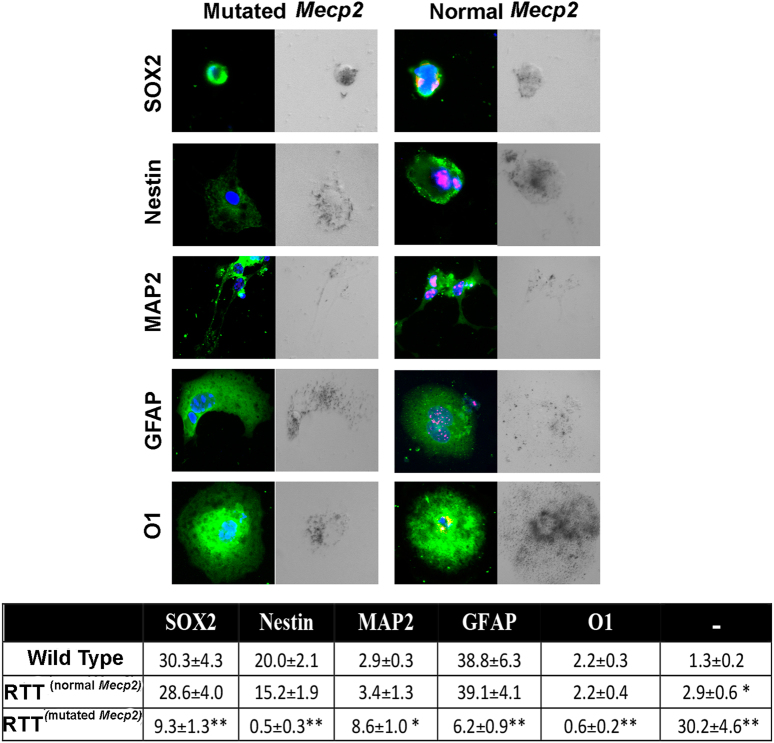
Representative photomicrographs of Mecp2+/− and control NSCs differentiated for 3 days onto poly-l-ornithine-coated plates in medium without growth factors. The cells were stained for SOX2, Nestin, MAP2, GFAP, and O1. The nuclei were counterstained with DAPI
Mecp2+/− female mice show a mosaic expression of wild-type and the mutated gene due to X-chromosome inactivation. In these animals, we evaluated neurogenesis and gliogenesis in cells expressing either wild-type or the mutated gene. Indeed, the MECP2 protein is undetectable in cells expressing the mutated gene.
In Mecp2+/− samples, the cells expressing the wild-type gene showed a normal differentiation process (Fig. 5). The percentage of progenitors and differentiated cells was similar to that observed in the control animals. In contrast, the cells expressing the mutated gene showed a significant reduction in the percentage of stem cells. We noticed an increase in the percentage of neurons (MAP2+) and a decrease in astrocytes (GFAP+) and oligodendrocytes (01+). Notably, we observed a massive increase in the number of cells that were negative for both stem and differentiation markers (Fig. 5). Of note, in progenitors and differentiated cells expressing mutated Mecp2, we observed a very significant increase (almost twofold) in senescent cells compared with the controls (Fig. 5).
Fig. 5. NSC differentiation and senescence in cells expressing wild-type and mutated Mecp2.
Cells expressing mutated or wild-type Mecp2 were identified by using the anti-MECP2 primary antibody. In Mecp2+/− samples, the MECP2 protein (red) was present in cells expressing the wild-type allele and was undetectable in those expressing the mutated allele. The picture shows representative photomicrographs of Mecp2+/− and control NSCs differentiated for 3 days onto poly-l-ornithine-coated plates in medium without growth factors. The cells were stained in green for SOX2, Nestin, MAP2, GFAP, and O1. The nuclei were counterstained with DAPI (blue). Every microscopic field was also analyzed under a bright-light field to detect senescent-associated β-galactosidase. The senescent cells show a dark gray staining. In the table are indicated the percentages of stem cells (SOX2+), early progenitors (Nestin+), neurons (MAP2+), astrocytes (GFAP+), and oligodendrocytes (O1+); the (−) symbol indicates the cells that were negative for all the analyzed markers. In the Mecp2+/− samples, indicated as RTT, the percentage of progenitors and differentiated cells was evaluated in cells expressing either the normal or the mutated allele (n = 5 biological replicates; *p < 0.05, **p < 0.01 with respect to samples from wild-type animals)
Discussion
Factors affecting chromatin status are key players in gene expression governance; hence, their deregulation has a great impact on cellular functions and may lead to adverse events, such as cellular transformation, apoptosis, or senescence. Up to 90% of patients with Rett syndrome have a mutation in the MECP2 gene, whose protein product is a chromatin remodeler that acts by binding methylated cytosines7. In previous investigations, we demonstrated that impaired MECP2 expression is associated with senescence in mesenchymal stromal cells8–10. In the current analysis, we aimed to evaluate whether the senescence phenomena could affect the biology of neural stem cells by altering cell commitment and/or differentiation in neural stem cells with reduced MECP2 activity. The rationale of this investigation resides on the consideration that, although MECP2 is expressed in the majority of the body tissues, in Rett patients, the great majority of the disease’s symptoms are neurological6,18,19.
Our study clearly demonstrated that the reduced expression of MECP2 is connected with an increase in senescence, a reduced proliferation capacity, and an accumulation of unrepaired DNA foci, of which this last issue is particularly intriguing. Indeed, we observed that the Mecp2+/− NSCs did not cope with genotoxic stress in the same way as the control cells did. In fact, following treatment with different DNA-damaging agents, the NSCs from mice with mutated Mecp2 accumulated more DNA damaged foci (γ-H2AX+) and were more prone to cell death than the controls. Following treatment with DNA stressors, the onset of cell death in Mecp2+/− NSC cultures, instead of entering into senescence, is a sign of reduced ability to counteract genotoxic events. Senescent cells are still live cells, while necrotic or apoptotic cells are dead.
The propensity of Mecp2+/− cells to accumulate DNA damage is consistent with several reports showing that abnormalities in chromatin remodeling, as may occur with reduced MECP2 expression, may alter the DNA repair process20,21. In cells with mutated MECP2, we detected a reduction of autophagic flux, in addition to the senescence phenomena and a reduced capacity to repair DNA. Impairment of autophagy may further contribute to the progressive deterioration of cell functions. Considering all of these observations together may explain, although speculatively, why patients with Rett syndrome develop normally for approximately 7 to 18 months after birth and then show progressive neurological regression6,22. On a daily basis, cells are subjected to intrinsic and extrinsic stresses and therefore must have an efficient DNA repair mechanism, which, after a genotoxic event, promotes the complete recovery of cells rather than triggering senescence or cell death. In Mecp2+/− cells, this capacity is impaired and may contribute to a rapid neurological decline.
Consistent with this are the results on the impairment of the neural differentiation process. In a previous work, we suggested that neural cell fate and neuronal maintenance could be perturbed by senescence triggered by impaired MECP2 activity8. That study was carried out on mesenchymal stromal cells obtained from Rett patients and on human neuroblastoma cells with a silenced MECP2. The two models were used to study neurogenesis with impaired MECP2 function. Another research group used normal and mutant MECP2-expressing human-induced pluripotent stem cell lines to study neurogenesis and also identified a deregulation of neurogenesis, with a bias toward astrocytes differentiation in the mutated samples23.
The limit of these studies was that a complete analysis of neural cell commitment and differentiation has to be performed with neural stem cells. Clearly, it is not possible to obtain NSCs from living RTT patients. In the current finding, we overcame such a limitation and utilized NSCs from a mouse model of Rett syndrome. Our data validated the previous hypothesis and demonstrated that reduced expression of MECP2 induces a decrease in cell proliferation and triggers the senescence of NSCs. This, in turn, reduced the number of stem cells and progenitors and gave rise to a high percentage of cells that expressed neither stem/progenitor nor differentiation markers. These may represent senescent non-functional cells.
Our study suggests that alteration of neurogenesis and gliogenesis in Mecp2+/− animals is a cell autonomous phenomenon. Indeed, in Mecp2+/− cultures, the cells expressing the wild-type gene showed a normal differentiation process, while those that expressed the mutated allele showed impaired neurogliogenesis.
Currently, there are no definitive treatments for RTT syndrome. This is because the physio-pathological consequences of the Mecp2 mutation are not well defined. Our research demonstrated that senescence of the stem cell compartments may be associated with impaired function of Mecp2. It would be reasonable to set up anti-aging treatments in an animal model of the RTT disease. If one of these treatments was proven effective in reducing the RTT-like symptoms in animal models, our research could pave the way to an innovative therapeutic approach for treating Rett syndrome.
Acknowledgements
This work was partially supported by the Ministry of Foreign Affairs, Republic of Italy, to U.G. (title of financed project: Senescence of stem cells and Rett syndrome). We thank Dr. Monica Mattia, Salvatore Esca, and Dr. Diego Di Nucci for animal care and handling.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nicola Alessio, Francesco Riccitiello.
References
- 1.Ozkul Y, Galderisi U. The impact of epigenetics on mesenchymal stem cell biology. J. Cell. Physiol. 2016;231:2393–2401. doi: 10.1002/jcp.25371. [DOI] [PubMed] [Google Scholar]
- 2.Di Bernardo G, Cipollaro M, Galderisi U. Chromatin modification and senescence. Curr. Pharm. Des. 2012;18:1686–1693. doi: 10.2174/138161212799859693. [DOI] [PubMed] [Google Scholar]
- 3.Antequera F. Structure, function and evolution of CpG island promoters. Cell. Mol. Life. Sci. 2003;60:1647–1658. doi: 10.1007/s00018-003-3088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuks F, et al. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J. Biol. Chem. 2003;278:4035–4040. doi: 10.1074/jbc.M210256200. [DOI] [PubMed] [Google Scholar]
- 5.Kimura H, Shiota K. Methyl-CpG-binding protein, MeCP2, is a target molecule for maintenance DNA methyltransferase Dnmt1. J. Biol. Chem. 2003;278:4806–4812. doi: 10.1074/jbc.M209923200. [DOI] [PubMed] [Google Scholar]
- 6.Smeets EE, Pelc K, Dan B. Rett Syndrome. Mol. Syndromol. 2012;2:113–127. doi: 10.1159/000337637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaving LS, Ellaway CJ, Gecz J, Christodoulou J. Rett syndrome: clinical review and genetic update. J. Med. Genet. 2005;42:1–7. doi: 10.1136/jmg.2004.027730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Squillaro T, et al. Reduced expression of MECP2 affects cell commitment and maintenance in neurons by triggering senescence: new perspective for Rett syndrome. Mol. Biol. Cell. 2012;23:1435–1445. doi: 10.1091/mbc.e11-09-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Squillaro T, et al. Partial silencing of methyl cytosine protein binding 2 (MECP2) in mesenchymal stem cells induces senescence with an increase in damaged DNA. FASEB J. 2010;24:1593–1603. doi: 10.1096/fj.09-143057. [DOI] [PubMed] [Google Scholar]
- 10.Squillaro T, et al. A case report: bone marrow mesenchymal stem cells from a Rett syndrome patient are prone to senescence and show a lower degree of apoptosis. J. Cell. Biochem. 2008;103:1877–1885. doi: 10.1002/jcb.21582. [DOI] [PubMed] [Google Scholar]
- 11.Bonaguidi MA, et al. Noggin expands neural stem cells in the adult hippocampus. J. Neurosci. 2008;28:9194–9204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jori FP, et al. RB and RB2/P130 genes cooperate with extrinsic signals to promote differentiation of rat neural stem cells. Mol. Cell. Neurosci. 2007;34:299–309. doi: 10.1016/j.mcn.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 14.Gary RK, Kindell SM. Quantitative assay of senescence-associated beta-galactosidase activity in mammalian cell extracts. Anal. Biochem. 2005;343:329–334. doi: 10.1016/j.ab.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Katz DM, et al. Preclinical research in Rett syndrome: setting the foundation for translational success. Dis. Model Mech. 2012;5:733–745. doi: 10.1242/dmm.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campisi J, d’Adda, di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 17.Mizushima N. Methods for monitoring autophagy. Int. J. Biochem. Cell Biol. 2004;36:2491–2502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Bienvenu T, Chelly J. Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized. Nat. Rev. Genet. 2006;7:415–426. doi: 10.1038/nrg1878. [DOI] [PubMed] [Google Scholar]
- 19.Singh J, Saxena A, Christodoulou J, Ravine D. MECP2 genomic structure and function: insights from ENCODE. Nucleic Acids Res. 2008;36:6035–6047. doi: 10.1093/nar/gkn591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price BD, D’Andrea AD. Chromatin remodeling at DNA double-strand breaks. Cell. 2013;152:1344–1354. doi: 10.1016/j.cell.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo G, et al. DNA damage and repair modify DNA methylation and chromatin domain of the targeted locus: mechanism of allele methylation polymorphism. Sci. Rep. 2016;6:33222. doi: 10.1038/srep33222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samaco RC, Neul JL. Complexities of Rett syndrome and MeCP2. J. Neurosci. 2011;31:7951–7959. doi: 10.1523/JNEUROSCI.0169-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andoh-Noda T, et al. Differentiation of multipotent neural stem cells derived from Rett syndrome patients is biased toward the astrocytic lineage. Mol. Brain. 2015;8:31. doi: 10.1186/s13041-015-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]