Abstract
Background
Although it is well established that heavy alcohol consumption increases the risk of hypertension, little is known about the effect of a reduction of alcohol intake on blood pressure. We aimed to assess the effect of a reduction in alcohol consumption on change in blood pressure stratified by initial amount of alcohol consumption and sex in adults.
Methods
In this systematic review and meta-analysis, we searched MedLine, Embase, CENTRAL, and ClinicalTrials. gov from database inception up to July 13, 2016, for trials investigating the effect of a change of alcohol consumption on blood pressure in adults using keywords and MeSH terms related to alcohol consumption, blood pressure, and clinical trials, with no language restrictions. We also searched reference lists of identified articles and published meta-analyses and reviews. We included full-text articles with original human trial data for the effect of a change of alcohol consumption on blood pressure in adults, which reported a quantifiable change in average alcohol consumption that lasted at least 7 days and a corresponding change in blood pressure. We extracted data from published reports. We did random-effects meta-analyses stratified by amount of alcohol intake at baseline. All meta-analyses were done with Stata (version 14.1). For the UK, we modelled the effect of a reduction of alcohol consumption for 50% of the population drinking more than two standard drinks per day (ie, 12 g pure alcohol per drink).
Findings
36 trials with 2865 participants (2464 men and 401 women) were included. In people who drank two or fewer drinks per day, a reduction in alcohol was not associated with a significant reduction in blood pressure; however, in people who drank more than two drinks per day, a reduction in alcohol intake was associated with increased blood pressure reduction. Reduction in systolic blood pressure (mean difference −5·50 mm Hg, 95% CI −6·70 to −4·30) and diastolic blood pressure (−3·97, −4·70 to −3·25) was strongest in participants who drank six or more drinks per day if they reduced their intake by about 50%. For the UK, the results would translate into more than 7000 inpatient hospitalisations and 678 cardiovascular deaths prevented every year.
Interpretation
Reducing alcohol intake lowers blood pressure in a dose-dependent manner with an apparent threshold effect. Implementation of effective alcohol interventions in people who drink more than two drinks per day would reduce the disease burden from both alcohol consumption and hypertension, and should be prioritised in countries with substantial alcohol-attributable risk.
Funding
National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (NIH).
Introduction
Hypertension (defined as systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg) affects more than 1 billion people worldwide and is projected to increase.1 Hypertension is the leading single risk factor for morbidity and mortality—responsible for 10·7 million deaths and 211·8 million disability-adjusted life-years worldwide in 2015.2 Similarly, alcohol consumption causes an enormous and growing global disease and economic burden3 despite a beneficial association of low alcohol consumption with ischaemic heart disease.4 Consequently, alcohol consumption and raised blood pressure are among the top five risk factors responsible for the growing global non-communicable diseases (NCD) burden,2 and are key parts of the WHO goals to reduce NCD mortality by 25% by 2025.5
The last review6 of the effects of alcohol reduction on blood pressure was done more than 15 years ago and showed an average systolic blood pressure reduction of −3·31 mm Hg (95% CI −4·10 to −2·52) when alcohol consumption was reduced; results were not presented by sex. Women and men metabolise alcohol differently because of differences in body fat distribution, body size, and alcohol solubility.7 With many more studies published since then, we did a systematic review and dose-response meta-analysis of trials assessing the effect of a reduction in alcohol consumption on change in blood pressure stratified by initial amount of alcohol consumption and sex in adults.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, following the PRISMA guidelines,8 we systematically searched MedLine, Embase, the Cochrane library (CENTRAL), and ClinicalTrials.gov, from inception to July 13, 2016, for trials investigating the effect of a change of alcohol consumption on blood pressure in adults using keywords and MeSH terms relating to alcohol consumption, blood pressure, and clinical trials (appendix pp 5–6). Additionally, we searched reference lists of identified articles and published meta-analyses and reviews. Inclusion criteria were as follows: full-text article with original human trial data for the effect of a change of alcohol consumption on blood pressure in adults; and trials that reported a quantifiable change in average alcohol consumption that lasted at least 7 days and a corresponding change in blood pressure. We did not apply language restrictions and authors were not contacted. MR and OSMH did the search and extraction, and conflicts were solved in discussion with a third reviewer (JR).
Data extraction
We abstracted study characteristics (appendix pp 3–4) including details of alcohol and blood pressure assessment (ambulatory blood pressure monitoring or office measurement [sitting, supine, standing]) and trial design (crossover or parallel-arm). We standardised alcohol intake before and after the intervention to the number of standard drinks with 12 g pure alcohol per drink per day.
We reported drinks rather than units to have the broadest global appeal. We included three articles from two randomised trials on initiating alcohol consumption in near abstainers.9–11 We labelled the high alcohol consumption period as baseline and the near abstention period as follow-up to make these trials comparable with all other trials. If a control group in a parallel-group trial reported a reduction in alcohol consumption, we included it as a “reduction of alcohol” group. Estimates of intervention effects by sex were preferred. Similarly, we preferred shorter time periods over longer time periods to avoid bias from potentially larger loss to follow-up. For the overall effect we preferred ambulatory blood pressure monitoring (24 h) over office blood pressure measurement when available. When only office blood pressure was reported, we preferred sitting, then supine, and then standing blood pressure measurement. Because of changing definitions of hypertension over time, we defined hypertension status at baseline as defined in the primary studies; or as taking antihypertensive drugs; or as mean systolic blood pressure at baseline as higher than 140 mm Hg. We defined mixed trials as not explicitly excluding people with hypertension or taking antihypertensive drugs. Trials that explicitly excluded people with diagnosed hypertension as defined above were classified as normotensive. Some trials provided analyses stratified by hypertension status, in which case we included each one separately in the analyses on the effect of hypertension status at baseline.
Data analysis
Recognising that quality score use in meta-analyses remains controversial,12,13 we did three complementary quality and risk of bias analyses. We classified trials based on the following criteria developed by the authors regarding technical aspects of clinical trials: more than 25 participants in each intervention group; randomisation of intervention procedures; washout periods of at least 1 week in crossover trials; reported results for a control group; attrition lower than 20%; no significant baseline differences between intervention groups; and a reported confidence interval, standard error, p value or F test for blood pressure change before and after intervention. Trials that fulfilled all seven criteria were labelled as high quality. We also did a risk of bias analysis for each study using the Cochrane Risk of Bias tool, which includes domains such as selection, performance, detection, and reporting bias.14 We rated the overall quality of evidence for a dose-response association using GRADE criteria, which are based on type of study design, consistency, magnitude, and dose-response gradients.15 Each study was included only once in each analysis, except in subgroup analyses in which hypertension status or blood pressure measurements were compared.
We pooled mean differences (MD, 95% CI) with inverse-variance weighting using DerSimonian-Laird random-effect models to allow for between-study heterogeneity.16 Small-study effects were examined using Egger’s regression-based test17 and visual inspection of funnel plots. Variation in the effect size because of between-study heterogeneity was quantified using the I2 statistic.18 Applying random-effects meta-regressions19 with a significance level p<0·1, we did analyses for the effect of: alcohol consumption at baseline in g per day, alcohol reduction from baseline to follow-up, hyper tension status at baseline categorised into people with hypertension, mixed populations, and normotensive people, crossover versus parallel trial design, length of the trial in weeks, office versus ambulatory blood pressure measurement, mean age, mean body-mass index (BMI), and high versus low trial quality. Analyses were done with Stata (version 14.1).
We used data from the UK to study the potential effect of population interventions for heavy alcohol use, assuming a coverage rate of 50% (ie, an intervention to reduce alcohol consumption for 50% of the population drinking more than two standard drinks per day), in line with the intervention rate for other interventions such as depression. We took the joint distributions of alcohol consumption and systolic blood pressure from the 2014 Health Survey of England,20 and the effect size of the intervention from the results of this meta-analysis. The exact modelling strategy has been described in detail elsewhere21 (appendix pp 2, 3). Analyses were done with Mathematica (version 10.4).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
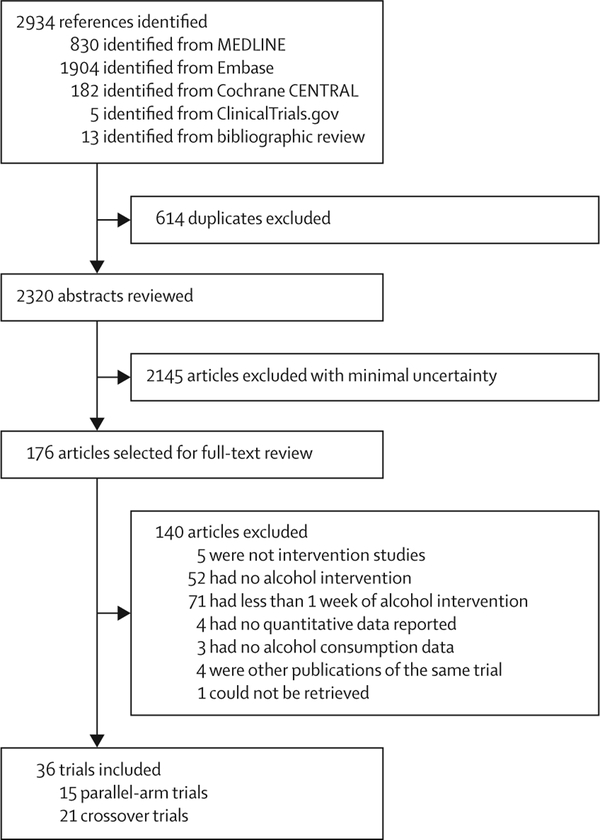
Of 2320 initial references, we reviewed 176 in full text (figure 1). For the systematic review, we used data from 36 articles including 2865 participants, with sex-specific data from 1413 men and 113 women.9–11,22–54 Overall, data from 2464 men and 401 women were analysed. Weighted mean age was 49·5 years and mean BMI was 27·1 kg/m2. 15 trials used a parallel-arm design,9–11,22–33 and 21 used a crossover design.34–54 13 trials provided data for people with hypertension (weighted mean systolic blood pressure 146 mm Hg), 12 had a mix of normotensives and hypertensives (weighted mean systolic blood pressure 137 mm Hg), and 13 trials provided data for normotensive people (weighted mean systolic blood pressure 122 mm Hg). The length of the trials ranged from 1 week to 2 years (median 4 weeks). Alcohol interventions ranged from controlled admin istration in a hospital setting to crossover trials with low alcohol content beverage substitution to pragmatic primary care trials with counselling to reduce consumption. We judged 12 studies from 11 trials to be of high technical quality.9–11,23,24,26,29,30,32,33,40,41 Only three trials presented data for women,28,50,53 making pooled effect estimates uncertain. One trial reported office blood pressure in one report,9 and ambulatory blood pressure monitoring in a much smaller substudy.10 Because of the much larger sample size, we used the trial with office blood pressure measurement9 for the main analyses, and the smaller substudy in a comparison of office and ambulatory blood pressure measurement. Trials were done in a range of high-income countries (table 1). The weighted mean alcohol consumption at baseline was 15, 30, 49, and 76 g per day in participants consuming two or fewer, three, four to five, and six or more drinks per day at baseline. The weighted mean reduction in alcohol consumption from baseline was −15, −30, −40, and −32 g per day in participants consuming two or fewer, three, four to five, and six or more drinks per day at baseline, respectively. Thus, alcohol at baseline was mostly compared with abstinence or near abstinence for people who drank five or fewer drinks per day at baseline, and about 50% mean reduction in alcohol intake for people who drank six or more drinks per day at baseline.
Figure 1: Study selection.
Table 1:
Trials of change in alcohol consumption and corresponding change in blood pressure meeting inclusion criteria
| Sex, age, country | Design, number of participants, and length of alcohol intervention | Inclusion and exclusion criteria | Alcohol intervention | Hypertension status at baseline | Blood pressure measurement | Quality assessment | ||
|---|---|---|---|---|---|---|---|---|
| Abe et al, 199448 | M, not reported, Japan | Crossover, n=14, 1 week | Essential hypertension with alcohol intake 30–120 mL per day; exclusion: secondary hypertension and cardiovascular, renal, hepatic, metabolic, and endocrine disorders | Hospital-based alcohol administration (1 mL/kg bodyweight, at dinner) vs non-alcoholic drinks (same calories, at dinner) | Hypertensive | 24 h | Low | |
| Aguilera et al, 199949 | M, 24–53 years, Spain |
Crossover, n=42, 4·5 weeks | Alcohol intake 100–380 g per day, admitted to the Alcohol Unit for voluntary alcohol detoxification | Hospital-based alcohol administration (total dose 2 g/kg) vs 1 month of abstinence (verified by interviews of relatives and GGT levels) | Normotensive | 24 h | Low | |
| Baros et al, 200822 | M/W, 44 years (mean), USA | Randomised parallel group, n=120, 12 weeks | Dependence on alcohol but not on other substances (except nicotine), no other major psychiatric diagnoses, medically stable, seeking outpatient treatment for alcoholism; liver enzymes (ALT, AST) less than 2·5 times the upper limit of normal | Naltrexone combined with either cognitive behavioural or motivational enhancement therapy for alcohol dependence; outcome: continued drinking vs abstinence | Mixed | Sitting | Low | |
| Chiva-Blanch et al, 201235 | M, 55–75 years, Spain | Randomised crossover, n=67, 4 weeks | Men at high cardiovascular risk (diabetes mellitus or ≥3 cardiovascular disease risk factors) | Common background diet plus red wine or gin (30 g alcohol per day) vs de-alcoholised red wine | Mixed, hypertensive and Normotensive subsamples |
Sitting | Low | |
| Cordain et al, 200050 | W, 30–50 years, USA | Randomised crossover, n=20, 10 weeks | Sedentary and overweight premenopausal women (BMI 27–33 kg/m2), alcohol intake two drinks per month to two drinks per week, willingness to consume two standard servings of red wine per day 5 days per week, for a total of 10 consecutive weeks; exclusion: health problems that can affect normal food intake and normal physical activity; use of any medications (including oral contraceptives) that can affect metabolism, appetite, or plasma lipids, glucose, and insulin; history of alcohol abuse or misuse; current alcohol intake greater than two standard servings per week; total avoidance of alcoholic beverages; use of supplemental omega-3 fatty acids; and participation in formal exercise more than two times per week | Red wine vs (near) abstinence | Normotensive | Sitting | Low | |
| Cox et al, 199323 | M, 20–45 years, Australia | Randomised parallel group, n=72, 4 weeks | Healthy, moderately drinking (≥210 mL per week), sedentary (<30 min vigorous-intensity exercise [energy expenditure >31·4 kJ/min] each week for 6 months before screening), BMI <30 kg/m2, systolic blood pressure 125–160 mm Hg, diastolic blood pressure <110 mm Hg | Usual alcohol intake vs reduced alcohol intake (substituted low alcohol beverages) | Mixed | Supine | High | |
| Cushman et al, 199824 | M/W, 21–79 years, USA | Randomised parallel group, n=549, 104 weeks | PATHS trial; outpatient veterans (average alcohol intake ≥three drinks per day), diastolic blood pressure 80–99 mm Hg; exclusion: alcohol or psychoactive substance dependence, alcohol-attributed medical complications, major psychiatric diagnoses, cardiovascular end-organ damage, severe or secondary hypertension, malignancies, seizure disorders, coagulopathies, or current pregnancy | Cognitive-behavioural alcohol reduction intervention programme or control observation; both groups reduced their alcohol consumption | Mixed, hypertensive subsample | Sitting | High | |
| Droste et al, 201330 | M/W, 63 years (mean), Luxembourg | Randomised parallel group, n=100, 20 weeks | Outpatients of the Department of Neurology and had undergone carotid and intra-cranial bitemporal colour-coded duplex sonography; inclusion criteria were age >30 years and the presence of plaques or stenosis without haemodynamic compromise (ie, <70%) in at least one common carotid artery, the carotid bifurcation or the internal carotid artery. Exclusion: history of ocular or cerebral ischaemia within the past 3 months, atrial fibrillation, a repeatedly measured systolic blood pressure >160 mm Hg | Common diet and exercise with red wine vs abstinence | Normotensive | 24 h | High | |
| Estruch et al, 201136 | M, 30–50 years, Spain | Randomised crossover, n=40, 4 weeks | Healthy men (alcohol intake 10–40 g per day) and no cardiovascular risk factors or receiving any medication or multivitamin or vitamin E supplements | Common diet and 30 g/day (red wine or gin) with dinner vs washout period (abstinence) | Normotensive | Office, details not reported | Low | |
| Flanagan et al, 200237 | M/W, 21–41 years, UK | Randomised crossover, n=21, 1 week | Healthy participants; exclusion: current diabetes or other current illness | Three units of alcohol daily for 1 week vs abstinence | Normotensive | Office, details not reported | Low | |
| Gepner et al, 20159 | M/W, 59 years (mean), Israel | Randomised parallel group, n=224, 104 weeks | Diagnosis of type 2 diabetes; exclusion: >one drink per week, personal or family history of addiction, smoking, stroke, or myocardial infarction; major surgery within the past 3 months; >two insulin injections per day or an insulin pump; triglyceride concentration >4·52 mmol/L (400 mg/dL), HbA1c level <6·4% or ≥10%; women with first-degree relatives with breast cancer; or pregnant women | White or red wine vs mineral water with dinner; beverages were provided | Mixed | Office, details not reported | High | |
| Gepner et al, 201610 | M/W, 57 years (mean), Israel | Randomised parallel group, n=54, 26 weeks | Age between 40 and 75 years, diagnosis of type 2 diabetes, alcohol abstainers (≤one drink per week), non-smokers, clinically stable, willingness to drink wine if so assigned by randomization, as part of a Mediterranean diet intervention | Common diet with dry red or white wine vs mineral water | Mixed | 24 h | High | |
| Hansen et al, 200525 | M/W, 38–75 years, Denmark | Randomised parallel group, n=69, 4 weeks | Healthy participant; exclusion: regular use of lipid lowering drugs, antihypertensives, and antioxidant supplements, uncommon dietary habits (eg, vegetarianism), and alcoholism. Major weight changes (43 kg) during intervention, elevated plasma concentrations (410 mg/L) of C-reactive protein | Red wine (men: 383 g alcohol/day, women: 255 g alcohol/day) vs water and grape extract tablets (wine-equivalent dose or half dose) or water and placebo tablets | Normotensive | Supine | Low | |
| Howes et al, 198638 | M, 18–35 years, UK | Randomised crossover, n=10, 1 week | Drinkers with less than 40 g per day usually | 0·8 g alcohol/kg bodyweight per day (taken between 1700 and midnight) vs abstinence | Normotensive | Supine | Low | |
| Hsieh et al, 199531 | M, 49 years (mean), Japan | Parallel, n=17, 4 weeks | Regular drinkers >40 g per day with untreated mild hypertension (sitting diastolic blood pressure 90–104 mm Hg ≥2 readings); exclusion: abnormal renal function, diabetes, serious liver dysfunction, known secondary causes of hypertension | Usual alcohol intake vs counselling to reduce alcohol intake as much as possible | Hypertensive | Supine | Low | |
| Kawano et al, 199839 | M, 36–76 years, Japan | Randomised crossover, n=34, 4 weeks | Habitually drinking (≥30 mL daily alcohol consumption) patients attending the Hypertension Clinic with essential hypertension | Usual drinking vs abstinence or reduced alcohol intake | Hypertensive | 24 h | Low | |
| Kawano et al, 199651 | M, 35–69 years, Japan | Crossover, n=16, 1 week | Mild-to-moderate hypertension; exclusion: serious cardiac, renal, or neurological disorders | Hospital-based alcohol administration (1 mL/kg bodyweight, at dinner) vs non-alcoholic drinks (same calories, at dinner) | Hypertensive | 24 h | Low | |
| Kim et al, 200934 | M/W, 30–65 years, USA | Crossover, n=20, 8 weeks | Insulin resistant, non-diabetic, not taking any medications known to affect carbohydrate metabolism; haematocrit >32%, ALT<2 times the upper limit of normal, and triglyceride concentration <4·5 mmol/L | 30 g/day (vodka or red wine) with dinner or before bedtime vs no alcohol itntake; beverages were provided | Normotensive | Office, details not reported | Low | |
| Lang et al, 199526 | M/W, 43 years (mean), France | Randomised parallel group, n=106, 104 weeks | Hypertensive (>140/90 mm Hg) and excessive drinkers (GGT >1·5 times normal); exclusion: planned departure or retirement in the next 2 years; diagnosis of secondary hypertension; severe liver disease (cirrhosis, alcoholic hepatitis, or alcohol related haemorrhage); high GGT not related to alcohol | Counselling to reduce alcohol intake (by trained physicians) vs continuing care (by physicians not trained); both groups reduced their alcohol consumption | Hypertensive | Sitting | High | |
| Maheswaran et al, 199227 | M, 44 years (mean), UK | Randomised parallel group, n=41, 8 weeks | Patients from hypertension clinic who regularly consumed more than 20 units of alcohol per week; exclusion: diastolic blood pressure exceeding 105 mm Hg at the time of recruitment, diabetes, known or suspected secondary causes of hypertension, diagnosed with alcoholism (problem with alcohol requiring referral to an alcohol addiction unit for admission and detoxification), having received advice previously and had reported reducing their alcohol consumption | Counselling to reduce alcohol intake vs no counselling | Hypertensive | Standing | Low | |
| Maiorano et al, 199552 | M, 46 years (mean), Italy | Crossover, n=15, 1 week | Normotensive men with history of heavy alcohol intake; exclusion: none | Hospital-based usual alcohol intake vs abstinence | Normotensive | 24 h | Low | |
| Mori et al, 201654 | M/W, 40–70 years, Australia | Randomised crossover, n=24, 4 weeks | Regular drinkers (men and postmenopausal women) with type 2 diabetes; women usually consumed 2–3 standard drinks per day (20–30 g per day) and men 3–4 standard drinks/day (30–40 g per day); exclusion criteria included type 1 diabetes, recent (<3 months) symptomatic heart disease, angina pectoris, history of myocardial infarction or stroke, peripheral vascular disease, major surgery 3 months or less, blood pressure >170/100 mm Hg, liver or renal disease (plasma creatinine >120 mmol/L), HbA1c >8·5% (>69 mmol/L), and current smokers or ex-smokers less than 2 years | Red wine vs equivalent volumes of dealcoholised red wine or water | Mixed | 24 h | Low | |
| Mori et al, 201553 | W, 24–45 years, Australia | Randomised crossover, n=24, 4 weeks | Regular, healthy, premenopausal, non-smoking drinkers. BMI <30 kg/m2, no history of hypertension, dyslipidaemia, diabetes mellitus, liver disease, or coronary, cerebrovascular or peripheral vascular disease, no clinical evidence of vascular disease, no medications (including aspirin, non-steroidal anti-inflammatory drugs, or the oral contraceptive pill) | Higher volume red wine (lower level drinkers, 146 g alcohol per week; higher level drinkers, 218 g alcohol per week) vs equal amounts of de-alcoholised red wine. Lower volume red wine (lower level drinkers, 42 g alcohol per week; higher level drinkers, 73 g alcohol per week) vs equal amounts of dealcoholised red wine | Normotensive | 24 h | Low | |
| Naissides et al, 200628 | W, 50–70 years, Australia | Randomised parallel group, n=45, 6 weeks | Moderately hypercholesterolaemic postmenopausal women; exclusion: hormone replacement therapy, lipid lowering medication, use of steroids and other agents that might influence lipid metabolism, use of warfarin, smoking, hyperthyroidism or hypothyroidism, diabetes mellitus, cardiovascular events within past 6 months, psychological unsuitability, major systemic diseases, gastrointestinal problems, proteinuria, liver and renal failure, apolipoprotein genotype (E2/E2 exclusion) | Common diet with red wine vs water or de-alcoholised red wine | Normotensive | Central | Low | |
| Parker et al, 199032 | M, 20–70 years, Australia | Randomised parallel group, n=59, 4 weeks | Stable, treated hypertension (systolic blood pressure ≥125–180, diastolic blood pressure <115 mm Hg), regular drinkers; regular treatment with antihypertensive drugs for at least the preceding 6 months, a minimum alcohol intake of 210 mL per week (about three standard drinks per day), no history of renal or hepatic disease or diabetes mellitus, not on current treatment with nonsteroidal anti-inflammatory drugs, and no history of a myocardial infarction, stroke, or coronary artery bypass surgery within the previous 12 months | Usual alcohol intake vs alcohol reduction (substitution with low alcohol content); beverages were provided | Hypertensive | Supine | High | |
| Puddey et al, 198540 | M, 25–55 years, Australia | Randomised crossover, n=46, 6 weeks | Healthy, normotensive regular drinkers (average alcohol intake ≥210 mL per week); exclusion: less than 210 mL alcohol per week, taking beta-blockers, chronic disease | Usual alcohol intake vs alcohol reduction (substitution with low alcohol content); beverages were provided | Normotensive | Supine | High | |
| Puddey et al, 198741 | M, 25–65 years, Australia | Randomised crossover, n=44, 6 weeks | Regular treatment with antihypertensive drugs for at least the preceding 6 months, minimum alcohol intake of 210 ml per week, no underlying renal disease | Usual alcohol intake vs alcohol reduction (substitution with low alcohol content); beverages were provided | Hypertensive | Supine | High | |
| Puddey et al, 199229 | M, 25–70 years, Australia | Randomised parallel group, n=86, 16 weeks | Overweight and moderately drinking men with minimum alcohol intake of 210 mL per week (about three standard drinks per day); body mass index of >25 kg/m2 or current weight greater than 120% of ideal weight for age; no current use of antihypertensive or nonsteroidal anti-inflammatory drugs; and no history of renal or hepatic disease, diabetes mellitus, myocardial infarction or coronary artery surgery, stroke, or substantial weight loss (>10 kg) in the preceding 12 months; blood pressure entry criteria (systolic blood pressure >130 mm Hg and <160 mm Hg, diastolic blood pressure >80 mm Hg and <105 mm Hg) | Usual alcohol intake vs alcohol reduction (substitution with low alcohol content); beverages were provided | Mixed | Supine | High | |
| Queipo-Ortuno et al, 201242 | M, 45–50 years, Spain | Randomised crossover, n=10, 3 weeks | Healthy, not receiving treatment for diabetes, hypertension, or dyslipidaemia, any acute or chronic inflammatory diseases, infectious diseases, viral infections, cancer, or a previous cardiovascular event at study entry, antibiotic therapy, prebiotics, probiotics, symbiotics, or vitamin supplements or any other medical treatment influencing intestinal microbiota during the 3 months before the study | Red wine or gin (30 g/day) vs de-alcoholised red wine or abstinence (initial washout period) | Mixed | Details not reported | Low | |
| Rakic et al, 199843 | M, 21–65 years, Australia | Randomised crossover, n=55, 4 weeks | Drinkers (210–500 mL alcohol per week [with >60% of total intake as beer]), no history of hypertension and no use of any drug affecting blood pressure, no liver, renal, and cardiovascular disorders and no hospitalisation for any medical or surgical illness during the preceding 3 months | Usual alcohol intake vs alcohol reduction (substitution with low alcohol content); beverages were provided | Normotensive | 24 h, supine | Low | |
| Shai et al, 200711 | M/W, 41–74 years, Israel | Randomised parallel group, n=91, 12 weeks | Type 2 diabetes, alcohol abstainers (≤one drink per week), non-smokers, clinically stable, and willingness to drink wine as part of a Mediterranean diet intervention. Exclusion: HbA1c <6.4% or >10%, insulin >2 injections/day or use of an insulin pump, fasting serum triglyceride ≥400 mg/dL, serum creatinine >2 mg/dL, liver dysfunction (≥3-fold increase in ALT and/or AST), evidence of severe diabetic complications (such as proliferative retinopathy or diabetic nephropathy), evidence of autonomic neuropathy manifesting as postural hypotension and/or hypoglycaemia unawareness, use of medications that might interact with moderate alcohol consumption, presence of active cancer and/or chemotherapy treatment in the past 3 years, presence of a major illness that might require hospitalisation, clinically assessed as having high potential of addictive behaviour or personal or family history of addiction or alcohol abuse, women with first degree relatives with breast cancer, pregnant or lactating women; and participation in another interventional trial | Common diet with initiation of alcohol intake (red or white wine with dinner) vs non-alcoholic diet malt beer with dinner; beverages were provided | Mixed | Sitting | High | |
| Ueshima et al, 199344 | M, 30–59 years, Japan | Randomised crossover, n=54, 3 weeks | Civil servants with systolic blood pressure >140 or diastolic blood pressure >90 mm Hg, more than 28 mL alcohol at least 4 times per week; exclusion: systolic blood pressure >179 or diastolic blood pressure >109 mm Hg, taking antihypertensive medication | Usual alcohol intake vs alcohol reduction (abstention or reduction as much as possible) | Hypertensive | Sitting | Low | |
| Ueshima et al, 198745 | M, 30–59 years, Japan | Randomised crossover, n=49, 2 weeks | Civil servants with blood pressure 140/90 mm Hg to 180/110 mm Hg; exclusion: diabetes with medication or less than 3 times a week alcohol consumption | Usual alcohol intake vs alcohol reduction (abstention or reduction as much as possible) | Hypertensive | Details not reported | Low | |
| Wallace et al, 198833 | M/W, 17–69 years, UK | Randomised parallel group, n=641, 52 weeks | Patients with excessive alcohol consumption (defined as at least 35 units/week in men and 21 units/week for women) | Common brief advice on general health (smoking, exercise, and diet) with counselling to reduce alcohol vs no counselling to reduce alcohol; both groups reduced their alcohol consumption | Mixed | Office, details not reported | High | |
| Zilkens et al, 200347 | M, 20–65 years, Australia | Randomised crossover, n=16, 4 weeks | Drinkers with 40–110 g/day, with more than 60% derived from beer; exclusion: smoking within the last 6 months, BMI >30, CVD (by clinical history, physical examination or electrocardiogram), diabetes mellitus, blood pressure >160/90 mm Hg or treatment with antihypertensive agents, total cholesterol >7.5 mmol/L or use of lipid-decreasing agents, aspirin or non-steroidal anti-inflammatory drugs | Usual alcohol intake vs alcohol reduction (substitution with low alcohol content); beverages were provided | Normotensive | Supine | Low | |
| Zilkens et al, 200546 | M, 39–65 years, Australia | Randomised crossover, n=24, 4 weeks | Regular drinkers with 30–60 g/day; exclusion: smoking within the last 6 months, BMI >30, cardiovascular disease (by clinical history, physical examination or electrocardiogram), diabetes, blood pressure >160/90 mm Hg or antihypertensive medication, total cholesterol >7.5 mmol/L or use of lipid-decreasing agents, aspirin or non-steroidal anti-inflammatory drugs | Red wine or beer vs abstinence or de-alcoholised red wine | Normotensive | 24 h | Low | |
M=men. W=women. M/W=men and women combined. M,W=men and women stratified. ALT=alanine aminotransaminase. AST=aspartate aminotransaminase. GGT=γ-glutamyl transferase. BMI=body-mass index. CVD=cardiovascular disease.
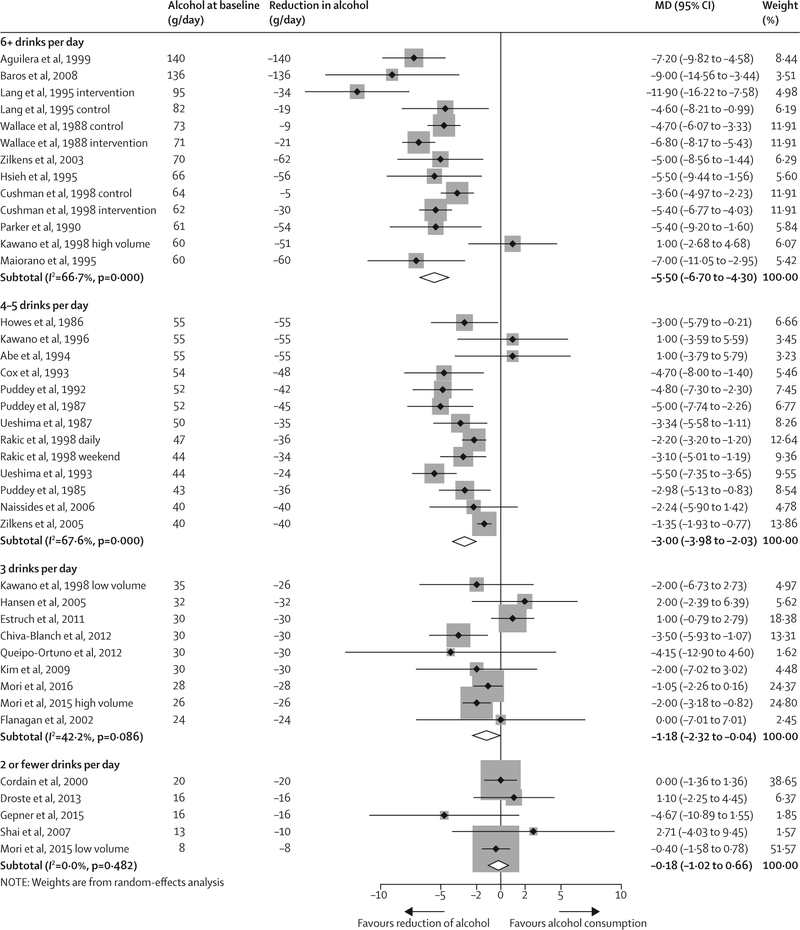
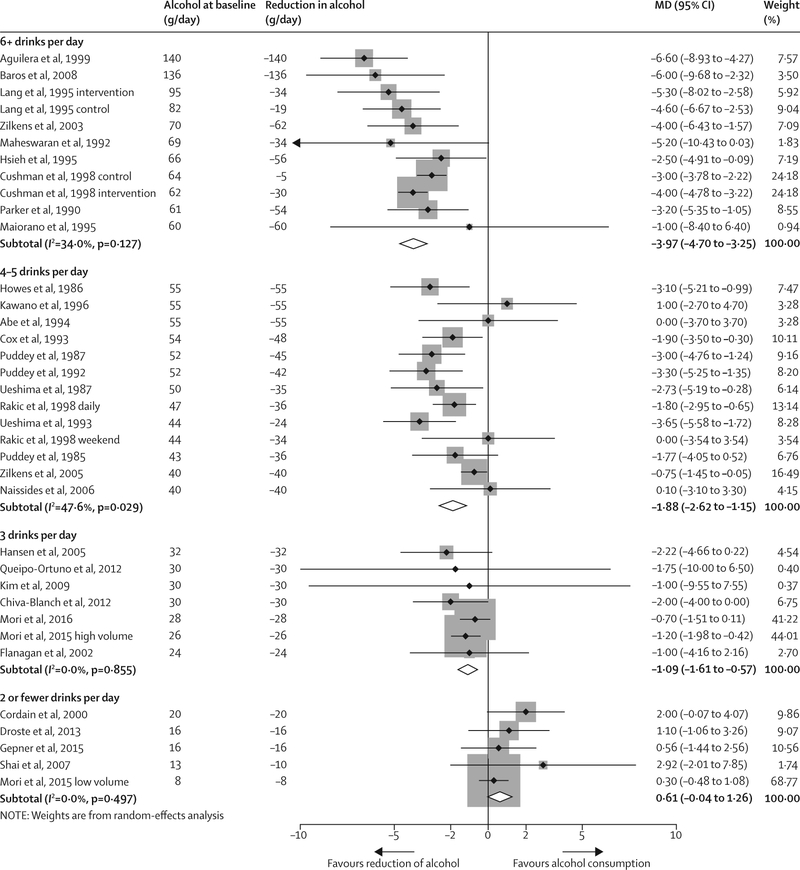
Figures 2 and 3 show the change in blood systolic and diastolic pressure stratified by alcohol consumption amount at baseline. The overall effect of a reduction of alcohol consumption across all trials was −3·13 mm Hg (95% CI −3·93 to −2·32) for systolic blood pressure and −2·00 (−2·65 to −1·35) for diastolic blood pressure with substantial between-study heterogeneity (I2 82·0% and 79·5%, respectively). In meta-regression models, the amount of alcohol intake from which consumption was reduced (mean alcohol at baseline) showed a strong effect on the magnitude of the blood pressure reduction (β = −0·91 mm Hg; p<0·0001 for systolic blood pressure; β = −0·75 mm Hg; p<0·0001 for diastolic blood pressure, per one drink per day). Alcohol intake at baseline explained 75·4% of the between-study variance in systolic blood pressure, and 93·4% in diastolic blood pressure.
Figure 2: Change in systolic blood pressure by alcohol consumption at baseline, all trials.
MD=mean difference in blood pressure (mm Hg). Weights (%) are the relative contribution of each study to the pooled mean difference in each initial drinking category.
Figure 3: Change in diastolic blood pressure by alcohol consumption at baseline, all trials.
MD=mean difference in blood pressure (mm Hg). Weights (%) are the relative contribution of each study to the pooled mean difference in each initial drinking category.
For people who drank two or fewer drinks per day, we recorded no significant effect of lower alcohol intake on pooled blood pressure (figures 2 and 3, table 2). A reduction of alcohol consumption to near abstinence for people who drank three drinks per day resulted in a significant change in systolic blood pressure (MD −1·18, 95% CI −2·32 to −0·04) and diastolic blood pressure (−1·09, −1·61 to −0·57). Reduction in systolic blood pressure (MD −5·50, 95% CI −6·70 to −4·30) and diastolic blood pressure (−3·97, −4·70 to −3·25) was strongest in participants who drank more than six drinks per day at baseline. Results were similar for men and women; however, data for women were sparse.28,50,53
Table 2:
Pooled effects of a reduction in alcohol consumption on blood pressure by baseline alcohol consumption
| Total | MD (95% CI) mm Hg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of trials* | Number of participants | Number of trials | ≤2 drinks per day | Number of trials | 3 drinks per day | Number of trials | 4–5 drinks per day | Number of trials | ≥6 drinks per day | |
| Systolic blood pressure | ||||||||||
| All trials | 34 | 2850 | 5 | −0·18 (−1·02 to 0·66) | 9 | −1.18 (−2.32 to −0.04) | 12 | −3.00 (−3.98 to 2.03) | 10 | −5.50 (−6.70 to 4.30) |
| Men only | 21 | 1413 | 0 | ·· | 4 | −1.59 (−4.60 to −1.42) | 11 | −3.04 (−4.07 to 2.02) | 7 | −5.26 (−6.79 to 3.73) |
| Women only | 3 | 113 | 2 | −0·23 (−1·12 to 0·66) | 1 | −2.00 (−3.18 to −0.82) | 1 | −2.24 (−5.90 to 1.42) | 0 | ·· |
| Hypertension only | 11 | 663 | 0 | ·· | 2 | −2.33 (−5.37 to 0.42) | 5 | −3.10 (−5.37 to 0.84) | 5 | −4.85 (−6.84 to 2.86) |
| Hypertension mixed | 9 | 1817 | 2 | −1·10 (−8·33 to 6·13) | 2 | −1.11 (−2.31 to 0.09) | 2 | −4.76 (−6.76 to 2.77) | 3 | −5.31 (−6.63 to 3.99) |
| Hypertension normo | 15 | 602 | 3 | −0·14 (−1·00 to 0·72) | 6 | −0.85 (−2.66 to 0.96) | 5 | −2.02 (−2.68 to 1.35) | 3 | −6.55 (−8.42 to 4.68) |
| Healthy | 15 | 1668 | 1 | −0·40 (−1·58 to 0·78) | 6 | −0.55 (−2.41 to 1.32) | 6 | −2.26 (−3.04 to 1.48) | 3 | −5.11 (−6.29 to 3.94) |
| High CVD risk | 16 | 1005 | 4 | 0·06 (−1·35 to 1·47) | 3 | −1.90 (−3.59 to −0.22) | 6 | −3.54 (−5.35 to 1.73) | 4 | −5.19 (−9.06 to 1.32) |
| High quality | 11 | 2264 | 3 | −0·02 (−3·79 to 3 75) | 1 | −2.00 (−6.73 to 2.73) | 4 | −4.17 (−5.46 to 2.88) | 5 | −5.06 (−6.60 to 3.52) |
| Crossover | 22 | 688 | 2 | −0·23 (−1·12 to 0·66) | 8 | −1.36 (−2.48 to −0.24) | 9 | −2.77 (−3.83 to 1.71) | 5 | −4.79 (−7.73 to 1.86) |
| Parallel-group | 12 | 2162 | 3 | −0·02 (−3·79 to 3 75) | 1 | 2.00 (−2.39 to 6.39) | 3 | −4.19 (−5.94 to 2.44) | 5 | −5.73 (−7.07 to 4.40) |
| Office | 24 | 2478 | 3 | −0.36 (−3·06 to 2·35) | 6 | −0.81 (−3.15 to 1.52) | 8 | −4.11 (−4.99 to 3.24) | 7 | −5.62 (−6.78 to 4.46) |
| 24 h | 10 | 372 | 2 | −0·24 (−1·34 to 0·87) | 3 | −1.55 (−2.39 to 0.72) | 4 | −1.74 (−2.62 to 0.86) | 3 | −4.45 (−9.65 to 0.74) |
| Weeks 1 to <3 | 6 | 125 | 0 | ·· | 1 | 0.00 (−7.01 to 7.01) | 4 | −1.88 (−4.00 to 0.24) | 1 | −7.00 (−11.05 to 2.95) |
| Weeks ≥3 to <10 | 20 | 888 | 2 | −0.24 (−1.34 to 0·87) | 8 | −1.21 (−2.42 to 0.00) | 7 | −3.16 (−4.33 to 1.99) | 5 | −4.52 (−7.31 to 1.72) |
| Weeks ≥10 | 8 | 1837 | 3 | −0.36 (−3.06 to 2·35) | 0 | ·· | 1 | −4.80 (−7.30 to 2.30) | 4 | −5.79 (−7.24 to 4.34) |
| Diastolic blood pressure | ||||||||||
| All trials | 32 | 2210 | 5 | 0·61 (−0.04 to 1·26) | 7 | −1.09 (−1.61 to 0.57) | 13 | −1.88 (−2.62 to 1.15) | 9 | −3.97 (−4.70 to 3.25) |
| Men only | 19 | 773 | 0 | ·· | 2 | −1.99 (−3.93 to −0.04) | 11 | −1.97 (−2.72 to 1.21) | 6 | −4.04 (−5.51 to 2.57) |
| Women only | 3 | 113 | 2 | 0·87 (−0.70 to 2·44) | 1 | −1.20 (−1.98 to 0.42) | 1 | 0.10 (−3.10 to 3.30) | 0 | ·· |
| Hypertension only | 11 | 704 | 0 | ·· | 1 | −1.00 (−3.77 to 1.77) | 5 | −2.29 (−3.75 to 0.82) | 5 | −3.61 (−4.20 to 3.01) |
| Hypertension mixed | 8 | 1176 | 2 | 0·89 (−0.96 to 2.74) | 2 | −0.71 (−1.52 to 0.10) | 2 | −2.48 (−3.84 to 1.13) | 2 | −3.67 (−4.70 to 2.65) |
| Hypertension normo | 15 | 562 | 3 | 0.73 (−0.21 to 1.66) | 5 | −1.31 (−2.04 to 0.63) | 5 | −1.32 (−2.12 to 0.53) | 3 | −4.91 (−7.36 to 2.45) |
| Healthy | 13 | 987 | 1 | 0.30 (−0.48 to 1.08) | 5 | −1.28 (−2.00 to 0.56) | 6 | −1.38 (−2.06 to 0.70) | 2 | −3.54 (−4.31 to 2.78) |
| High CVD risk | 16 | 1046 | 4 | 1.30 (0.14 to 2.47) | 2 | −1.01 (−2.11 to 0.08) | 6 | −2.58 (−3.74 to 1.42) | 4 | −3.91 (−5.03 to 2.79) |
| High quality | 10 | 1583 | 3 | 0.98 (−0.43 to 2.39) | 0 | ·· | 4 | −2.50 (−3.42 to 1.57) | 3 | −3.69 (−4.40 to 2.98) |
| Crossover | 20 | 648 | 2 | 0.87 (−0.70 to 2.44) | 6 | −1.04 (−1.57 to 0.50) | 9 | −1.84 (−2.70 to 0.97) | 4 | −4.13 (−6.33 to 1.94) |
| Parallel-group | 12 | 1562 | 3 | 0.98 (−0.43 to 2.39) | 1 | −2.22 (−4.66 to 0.22) | 3 | −2.04 (−3.61 to 0.46) | 5 | −3.79 (−4.46 to 3.11) |
| Office | 23 | 1893 | 3 | 1.38 (0.00 to 2.77) | 5 | −1.85 (−3.21 to 0.50) | 9 | −2.32 (−2.93 to 1.71) | 7 | −3.67 (−4.21 to 3.13) |
| 24 h | 8 | 317 | 2 | 0.39 (−0.34 to 1.13) | 2 | −0.96 (−1.52 to 0.39) | 3 | −0.67 (−1.34 to 0.01) | 2 | −4.95 (−9.95 to 0.06) |
| Weeks 1 to <3 | 6 | 125 | 0 | ·· | 1 | −1.00 (−4.16 to 2.16) | 4 | −1.71 (−3.56 to 0.14) | 1 | −1.00 (−8.40 to 6.40) |
| Weeks ≥3 to <10 | 19 | 889 | 2 | 0.39 (−0.34 to 1.13) | 6 | −1.09 (−1.62 to 0.57) | 7 | −1.73 (−2.57 to 0.89) | 5 | −4.15 (−5.70 to 2.61) |
| Weeks ≥10 | 7 | 1196 | 3 | 1.38 (0.00 to 2.77) | 0 | ·· | 1 | −3.30 (−5.25 to 1.35) | 3 | −3.92 (−4.79 to 3.05) |
1 drink=12 g pure alcohol. CVD=cardiovascular disease. *Might not add up because some trials reported results for more than one drinking group.
In analyses of systolic blood pressure, aside from the strong effect of alcohol intake at baseline, length of the trial (β −0·036 mm Hg per week; p=0·042) and blood pressure assessment method (24 h vs office, β −1·14 mm Hg; p=0·043) were significantly associated with blood pressure reduction in multivariate meta-regression models when alcohol at baseline was controlled for. However, the improvement of explained variance was small (3%). Findings of subgroup analyses showed that the strong dose-response association for amount of alcohol intake at baseline was found in all strata (table 2). Exclusion of three trials24,26,33 in which the control group reduced its alcohol consumption had almost no effect on the findings. Similarly, healthy participants showed the same dose-response association for initial alcohol intake reported in all other subgroup analyses. No trial characteristics other than alcohol at baseline were significantly associated with diastolic blood pressure change and there was little heterogeneity left to explain (I2=15·4%). Of 27 trials that reported on weight change during alcohol reduction, only ten reported a significant change (between −0·3 and −1·7 kg), the other trials reported no significant or no change in weight.
We found no evidence for small-study effects or publication bias (Egger’s test; p=0·10 and 0·47 for systolic blood pressure and diastolic blood pressure, respectively). Leaving each trial out of the analysis one at a time showed no meaningful differences in effects (appendix pp 8–11). The Cochrane Risk of Bias method showed potential high risk of bias from masking and allocation concealment, and low or unclear risk in most other domains (appendix p 12). Using the GRADE approach, we rate the quality of the evidence for a dose-response association as high because of the consistency, precision, and magnitude of the effects based on randomised controlled trial data, with high clinical importance in heavy drinkers.
Table 3 gives an estimate of the effect of a reduction of alcohol intake for half of the people in the UK general population drinking more than two drinks per day. We assumed the effect size based on data from table 2, specific for sex and amount of alcohol consumption. Overall, the proportion of people with systolic blood pressure higher than 140 mm Hg was estimated to fall by 4·4% for men, and 1·2% for women, with most of the effect emerging in mid-adulthood. This reduction of blood pressure translates into marked clinical effects, with 7272 inpatient hospitalisations (1207 in women and 6064 in men) and 678 cardiovascular disease (CVD) deaths avoided every year (125 in women, 552 in men), mainly in ischaemic heart disease (appendix p 7).
Table 3:
Estimations of the proportion of people with systolic blood pressure >140 mm Hg before and after a reduction in alcohol intake by sex and age in the UK, 2014
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Population | Before | After | Proportional difference | Population | Before | After | Proportional difference | |
| Age 15–34 years | 8 206 000 | 14.6% | 13.8% (1 132 428) | −5.5% | 7 899 000 | 6.0% | 5.9% (466 041) | −1.7% |
| Age 35–64 years | 12 210 000 | 27.9% | 26.4% (3 223 440) | −5.4% | 12 560 000 | 17.1% | 16.7% (2 097 520) | −2.3% |
| Age >65 years | 4 529 000 | 40.2% | 39.1% (1 770 839) | −2.7% | 5 767 000 | 41.1% | 40.9% (2 358 703) | −0.5% |
| All | 24 945 000 | 25.7% | 24.6% (6 126 707) | −4.4% | 26 226 000 | 19.0% | 18.8% (4 922 264) | −1.2% |
The joint distributions of alcohol consumption and systolic blood pressure were taken from the Health Survey of England 2014.20 The effect size of the intervention was taken from the results of this meta-analysis. Details of the modelling strategy have been described elsewhere21 and are summarised in the appendix pp 2–3.
Discussion
A reduction in alcohol consumption reduced blood pressure in a dose-dependent manner with an apparent threshold effect at two drinks per day. People drinking two drinks or fewer per day did not have a significant reduction in blood pressure when they reduced their alcohol consumption to near abstinence, suggesting that this amount of alcohol intake does not increase blood pressure. However, the more people drink beyond this level, the higher the subsequent reduction in blood pressure. The dose-response association was evident in healthy participants and people with hypertension or other CVD risk factors. The blood pressure reduction is similar to that of other health behaviour changes, such as physical activity,55 weight loss diets,56 or general behavioural counselling including diet and physical activity.57
Like any meta-analysis, our findings are only as good as the underlying primary trials. Although there was initially substantial between-study heterogeneity, the amount of alcohol consumption at baseline explained almost all of this heterogeneity. There was no evidence for small-study effects or any overly influential single trial. The modelling of the effects on a population level was based on standard methods for comparative risk assessment analyses (eg, Global Burden of Disease studies)2,58 assuming persistence of lower alcohol consumption within 1 year, and no lag time of effects on CVD outcomes. However, although length of the trial was associated with the effect size, findings of subgroup analyses showed a strong dose response association for initial alcohol consumption independent of the length of the trial. Three trials provided multiple blood pressure measurements over the length of the trial.24,26,30 There was almost no difference from the first measurement to the last, which suggests that the effect of a reduction in alcohol consumption on blood pressure is sustained when lower alcohol consumption is sustained. Nevertheless, increasing attrition in longer trials suggests a possible risk of bias and there is a need to investigate how alcohol interventions should best be applied to reduce blood pressure. Some studies found a small change in bodyweight during the trial, which is expected because less alcohol is consumed and the weight change is probably part of an intermediate pathway of the effect of an alcohol reduction on blood pressure.
Because only three trials reported results for women,28,50,53 we have less confidence in the pooled effect estimates. Similarly, there was only one trial in people with hypertension who consumed three or fewer drinks per day. Because of the public health importance of both alcohol consumption and hypertension, there is an urgent need for additional research to clarify the effect of alcohol intake in people with hypertension at low alcohol intake and in women.
With regard to risk of bias, masking and allocation concealment are almost impossible to achieve in trials investigating lifestyle changes such as alcohol consumption. The high quality trials as defined by our criteria were consistent with a dose-response association recorded in all subgroup analyses. Because of this consistency, and the precision and magnitude of the effects, we rate the quality of evidence for a dose-response association as high. Moreover, the consistency of the dose-response association subgroups defined by CVD risk, study design, and study quality suggests that our findings should have high generalisability. Despite the evidence from randomised trial data presented here and also from observational data,59 the physiological mechanisms for alcohol’s effect on blood pressure and hypertension are still unknown.60
From a public health perspective, both alcohol consumption and raised blood pressure are among the most important risk factors for the global burden of NCDs.61 A reduction of both alcohol consumption and blood pressure has the potential for substantial synergistic health gains in terms of morbidity, mortality, and health-care costs; yet only about half of hypertension guidelines worldwide recommend a reduction in alcohol consumption to reduce raised blood pressure.62 Aside from the substantial estimated effect on CVD mortality and morbidity caused by hypertension, a reduction of alcohol consumption has additional effects on disease burden63 not modelled here. This would be an important contribution to reaching the goals of the WHO Global Action Plan for the prevention of NCDs,5 which stipulates a 10% relative reduction of harmful alcohol use and a 25% reduction in raised blood pressure by 2025 to reduce NCD mortality by 25%. Thus, screening for alcohol consumption and application of brief interventions to reduce hazardous and harmful alcohol consumption, or referral to treatment for more severe cases,64 should be a priority in primary health care. Similarly, awareness and treatment for hypertension are not optimal,65 and might be especially important in heavy drinkers.66 For heavy drinkers, a reduction in alcohol consumption to two or fewer drinks per day could be the first choice in treatment of hypertension. In terms of alcohol policy, price increase and availability restrictions have been shown to be effective and cost-effective interventions to reduce harmful alcohol consumption on a population level.67
In conclusion, a reduction of alcohol intake reduces blood pressure in a dose-dependent manner with a possible threshold effect. The identification of people who drink more than two drinks per day and implementation of effective alcohol interventions would substantially reduce the disease burden from both alcohol and raised blood pressure, and should be prioritised in research and primary care in countries with a substantial alcohol-attributable disease burden to prevent and reduce NCD burden in line with WHO goals for 2025.
Supplementary Material
Research in context
Evidence before this study
Findings of randomised controlled trials and observational studies have shown that heavy alcohol consumption increases blood pressure and the incidence of hypertension. The last review on reduction of alcohol consumption and blood pressure change is more than 15 years old. The effects of lowering alcohol intake over a range of initial alcohol consumption levels have not been quantified systematically. We searched MedLine, Embase, the Cochrane library (CENTRAL), and ClinicalTrials.gov, from database inception to July 13, 2016, for trials assessing the dose-response association between reduced alcohol consumption and subsequent changes in systolic and diastolic blood pressure using keywords and MeSH terms related to alcohol consumption, blood pressure, and clinical trials. Additionally, we searched reference lists of identified articles and published meta-analyses and reviews. Inclusion criteria were as follows: full-text article with original human trial data for the effect of a change of alcohol consumption on blood pressure in adults; and trials that reported a quantifiable change in average alcohol consumption that lasted at least 7 days and a corresponding change in blood pressure. We did not apply language restrictions and authors were not contacted.
Added value of this study
In people who drank two or fewer drinks per day (12 g pure alcohol per drink), a reduction in alcohol intake was not associated with a significant reduction in blood pressure. In people who drank at least three drinks per day, a reduction of alcohol consumption to near abstinence was associated with a reduction in blood pressure. Reductions in systolic blood pressure and diastolic blood pressure were strongest in participants who drank six or more drinks per day for a 50% reduction in alcohol intake. As per our meta-analysis findings, we estimate that more than 7000 inpatient hospitalisations and 678 cardiovascular deaths caused by hypertension would be prevented per year in the UK if people who drank more than two drinks per day reduced their alcohol consumption.
Implications of all the available evidence
Alcohol consumption and raised blood pressure are among the most important risk factors for non-communicable diseases. A reduction of both alcohol consumption and blood pressure has the potential for substantial synergistic health gains and health-care costs. Identification and implementation of effective alcohol interventions in people who drink more than two drinks per day could substantially reduce the disease burden from raised blood pressure and should be prioritised.
Acknowledgments
This study was funded by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R21AA023521 to MR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interests
MR and JR report grants from National Institutes of Health (NIH), National Institute on Alcohol Abuse and Alcoholism (NIAAA), during the conduct of the study. JR reports grants and personal fees from Lundbeck outside of this work. JK, SWT, GG, and OSMH declare no competing interests.
References
- 1.WHO. A global brief on hypertension: silent killer, global public health crisis. http://www.thehealthwell.info/node/466541 (accessed July 23, 2016).
- 2.GBD 2013 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1659–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Global status report on alcohol and health. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 4.Roerecke M, Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Medicine 2014; 12: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Global status report on noncommunicable diseases 2014. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 6.Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 2001; 38: 1112–17. [DOI] [PubMed] [Google Scholar]
- 7.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med 1990; 322: 95–99. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–69. [DOI] [PubMed] [Google Scholar]
- 9.Gepner Y, Golan R, Harman-Boehm I, et al. Effects of initiating moderate alcohol intake on cardiometabolic risk in adults with type 2 diabetes: a 2-year randomized, controlled trial. Ann Intern Med 2015; 163: 569–79. [DOI] [PubMed] [Google Scholar]
- 10.Gepner Y, Henkin Y, Schwarzfuchs D, et al. Differential effect of initiating moderate red wine consumption on 24-h blood pressure by alcohol dehydrogenase genotypes: randomized trial in type 2 diabetes. Am J Hypertens 2016; 29: 476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shai I, Wainstein J, Harman-Boehm I, et al. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes: a multicenter, randomized, clinical intervention trial. Diabetes Care 2007; 30: 3011–16. [DOI] [PubMed] [Google Scholar]
- 12.Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics 2001; 2: 463–71. [DOI] [PubMed] [Google Scholar]
- 13.Herbison P, Hay-Smith J, Gillespie WJ. Adjustment of meta-analyses on the basis of quality scores should be abandoned. J Clin Epidemiol 2006; 59: 1249–56. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.handbook.cochrane.org (accessed July 7, 2016).
- 15.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–06. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 19.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002; 21: 1559–73. [DOI] [PubMed] [Google Scholar]
- 20.Health and Social Care Information Centre. Health Survey for England, 2014. http://www.hscic.gov.uk/catalogue/PUB19295 (accessed July 20, 2016).
- 21.Rehm J, Gmel G, Sierra C, Gual A. Reducing non-communicable disease via better detection of hypertension and alcohol problems in primary health care in Spain. Addiciones 2016; published online Sept 29. DOI:DOI: 10.20882/adicciones.726. [DOI] [Google Scholar]
- 22.Baros AM, Wright TM, Latham PK, Miller PM, Anton RF. Alcohol consumption, %CDT, GGT and blood pressure change during alcohol treatment. Alcohol Alcohol 2008; 43: 192–97. [DOI] [PubMed] [Google Scholar]
- 23.Cox KL, Puddey IB, Morton AR, Beilin LJ, Vandongen R, Masarei JR. The combined effects of aerobic exercise and alcohol restriction on blood pressure and serum lipids: a two-way factorial study in sedentary men. J Hypertens 1993; 11: 191–201. [DOI] [PubMed] [Google Scholar]
- 24.Cushman WC, Cutler JA, Hanna E, et al. Prevention and Treatment of Hypertension Study (PATHS): effects of an alcohol treatment program on blood pressure. Arch Intern Med 1998; 158: 1197–207. [DOI] [PubMed] [Google Scholar]
- 25.Hansen AS, Marckmann P, Dragsted LO, Finne Nielsen IL, Nielsen SE, Gronbaek M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. Eur J Clin Nutr 2005; 59: 449–55. [DOI] [PubMed] [Google Scholar]
- 26.Lang T, Nicaud V, Darne B, RueffB. Improving hypertension control among excessive alcohol drinkers: a randomised controlled trial in France. The WALPA Group. J Epidemiol Community Health 1995; 49: 610–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maheswaran R, Beevers M, Beevers DG. Effectiveness of advice to reduce alcohol consumption in hypertensive patients. Hypertension 1992; 19: 79–84. [DOI] [PubMed] [Google Scholar]
- 28.Naissides M, Pal S, Mamo JCL, James AP, Dhaliwal S. The effect of chronic consumption of red wine polyphenols on vascular function in postmenopausal women. Eur J Clin Nutr 2006; 60: 740–45. [DOI] [PubMed] [Google Scholar]
- 29.Puddey IB, Parker M, Beilin LJ, Vandongen R, Masarei JR. Effects of alcohol and caloric restrictions on blood pressure and serum lipids in overweight men. Hypertension 1992; 20: 533–41. [DOI] [PubMed] [Google Scholar]
- 30.Droste DW, Iliescu C, Vaillant M, et al. A daily glass of red wine associated with lifestyle changes independently improves blood lipids in patients with carotid arteriosclerosis: results from a randomized controlled trial. Nutr J 2013; 12: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh ST, Saito K, Miyajima T, Lin CM, Yokoyama M. Effects of alcohol moderation on blood pressure and intracellular cations in mild essential hypertension. Am J Hypertens 1995; 8: 696–703. [DOI] [PubMed] [Google Scholar]
- 32.Parker M, Puddey IB, Beilin LJ, Vandongen R. Two-way factorial study of alcohol and salt restriction in treated hypertensive men. Hypertension 1990; 16: 398–406. [DOI] [PubMed] [Google Scholar]
- 33.Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ 1988; 297: 663–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SH, Abbasi F, Lamendola C, Reaven GM. Effect of moderate alcoholic beverage consumption on insulin sensitivity in insulin-resistant, nondiabetic individuals. Metabolism 2009; 58: 387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiva-Blanch G, Urpi-Sarda M, Ros E, et al. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: Short communication. Circ Res 2012; 111: 1065–68. [DOI] [PubMed] [Google Scholar]
- 36.Estruch R, Sacanella E, Mota F, et al. Moderate consumption of red wine, but not gin, decreases erythrocyte superoxide dismutase activity: a randomised cross-over trial. Nutr Metab Cardiovasc Dis 2011; 21: 46–53. [DOI] [PubMed] [Google Scholar]
- 37.Flanagan DE, Pratt E, Murphy J, et al. Alcohol consumption alters insulin secretion and cardiac autonomic activity. Eur J Clin Invest 2002; 32: 187–92. [DOI] [PubMed] [Google Scholar]
- 38.Howes LG, Reid JL. Changes in blood pressure and autonomic reflexes following regular, moderate alcohol consumption.J Hypertens 1986; 4: 421–25. [DOI] [PubMed] [Google Scholar]
- 39.Kawano Y, Abe H, Takishita S, Omae T. Effects of alcohol restriction on 24-hour ambulatory blood pressure in Japanese men with hypertension. Am J Med 1998; 105: 307–11. [DOI] [PubMed] [Google Scholar]
- 40.Puddey IB, Beilin LJ, Vandongen R. Evidence for a direct effect of alcohol consumption on blood pressure in normotensive men. A randomized controlled trial. Hypertension 1985; 7: 707–13. [DOI] [PubMed] [Google Scholar]
- 41.Puddey IB, Beilin LJ, Vandongen R. Regular alcohol use raises blood pressure in treated hypertensive subjects. A randomised controlled trial. Lancet 1987; 1: 647–51. [DOI] [PubMed] [Google Scholar]
- 42.Queipo-Ortuno MI, Boto-Ordonez M, Murri M, et al. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr 2012; 95: 1323–34. [DOI] [PubMed] [Google Scholar]
- 43.Rakic V, Puddey IB, Burke V, Dimmitt SB, Beilin LJ. Influence of pattern of alcohol intake on blood pressure in regular drinkers: a controlled trial. J Hypertens 1998; 16: 165–74. [DOI] [PubMed] [Google Scholar]
- 44.Ueshima H, Mikawa K, Baba S, et al. Effect of reduced alcohol consumption on blood pressure in untreated hypertensive men. Hypertension 1993; 21: 248–52. [DOI] [PubMed] [Google Scholar]
- 45.Ueshima H, Ogihara T, Baba S, et al. The effect of reduced alcohol consumption on blood pressure: a randomised, controlled, single blind study. J Hum Hypertens 1987; 1: 113–19. [PubMed] [Google Scholar]
- 46.Zilkens RR, Burke V, Hodgson JM, Barden A, Beilin LJ, Puddey IB. Red wine and beer elevate blood pressure in normotensive men. Hypertension 2005; 45: 874–79. [DOI] [PubMed] [Google Scholar]
- 47.Zilkens RR, Rich L, Burke V, Beilin LJ, Watts GF, Puddey IB. Effects of alcohol intake on endothelial function in men: a randomized controlled trial. J Hypertens 2003; 21: 97–103. [DOI] [PubMed] [Google Scholar]
- 48.Abe H, Kawano Y, Kojima S, et al. Biphasic effects of repeated alcohol intake on 24-hour blood pressure in hypertensive patients. Circulation 1994; 89: 2626–33. [DOI] [PubMed] [Google Scholar]
- 49.Aguilera MT, de la Sierra A, Coca A, Estruch R, Fernandez-Sola J, Urbano-Marquez A. Effect of alcohol abstinence on blood pressure: assessment by 24-hour ambulatory blood pressure monitoring. Hypertension 1999; 33: 653–57. [DOI] [PubMed] [Google Scholar]
- 50.Cordain L, Melby CL, Hamamoto AE, et al. Influence of moderate chronic wine consumption on insulin sensitivity and other correlates of syndrome X in moderately obese women. Metabolism 2000; 49: 1473–78. [DOI] [PubMed] [Google Scholar]
- 51.Kawano Y, Abe H, Kojima S, et al. Different effects of alcohol and salt on 24-hour blood pressure and heart rate in hypertensive patients. Hypertens Res 1996; 19: 255–61. [DOI] [PubMed] [Google Scholar]
- 52.Maiorano G, Bartolomucci F, Contursi V, Saracino E, Agostinacchio E. Effect of alcohol consumption versus abstinence on 24-h blood pressure profile in normotensive alcoholic patients. Am J Hypertens 1995; 8: 80–81. [DOI] [PubMed] [Google Scholar]
- 53.Mori TA, Burke V, Beilin LJ, Puddey IB. Randomized controlled intervention of the effects of alcohol on blood pressure in premenopausal women. Hypertension 2015; 66: 517–23. [DOI] [PubMed] [Google Scholar]
- 54.Mori TA, Burke V, Zilkens RR, Hodgson JM, Beilin LJ, Puddey IB. The effects of alcohol on ambulatory blood pressure and other cardiovascular risk factors in type 2 diabetes: a randomized intervention. J Hypertens 2016; 34: 421–28. [DOI] [PubMed] [Google Scholar]
- 55.Williamson W, Foster C, Reid H, et al. Will exercise advice be sufficient for treatment of young adults with prehypertension and hypertension? a systematic review and meta-analysis. Hypertension 2016; 68: 78–87. [DOI] [PubMed] [Google Scholar]
- 56.Semlitsch T, Jeitler K, Berghold A, et al. Long-term effects of weight-reducing diets in people with hypertension. Cochrane Database Syst Rev 2016; 3: Cd008274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin JS, O’Connor EA, Evans CV, Senger CA, Rowland MG, Groom HC. US Preventive Services Task Force evidence syntheses, formerly systematic evidence reviews behavioral counseling to promote a healthy lifestyle for cardiovascular disease prevention in persons with cardiovascular risk factors: an updated systematic evidence review for the US Preventive Services Task Force. Rockville, MD, USA: Agency for Healthcare Research and Quality, 2014. [PubMed] [Google Scholar]
- 58.Ezzati M, Lopez AD, Rodgers A, Murray CJL. Comparative quantification of health risks global and regional burden of disease attributable to selected major risk factors. Geneva: World Health Organization, 2004. [Google Scholar]
- 59.Taylor B, Irving HM, Baliunas D, et al. Alcohol and hypertension: gender differences in dose-response relationships determined through systematic review and meta-analysis. Addiction 2009; 104: 1981–90. [DOI] [PubMed] [Google Scholar]
- 60.Klatsky AL, Gunderson E. Alcohol and hypertension: a review. J Am Soc Hypertens 2008; 2: 307–17. [DOI] [PubMed] [Google Scholar]
- 61.WHO. Global Action plan for the prevention and control of noncommunicable diseases 2013–2020. Geneva: World Health Organization, 2013. [Google Scholar]
- 62.Chalmers J, Arima H, Harrap S, Touyz RM, Park JB. Global survey of current practice in management of hypertension as reported by societies affiliated with the International Society of Hypertension. J Hypertens 2013; 31: 1043–48. [DOI] [PubMed] [Google Scholar]
- 63.Rehm J, Roerecke M. Reduction of drinking in problem drinkers and all-cause mortality. Alcohol Alcohol 2013; 48: 509–13. [DOI] [PubMed] [Google Scholar]
- 64.Kaner EF, Beyer F, Dickinson HO, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2007; 2: Cd004148. [DOI] [PubMed] [Google Scholar]
- 65.Joffres M, Falaschetti E, Gillespie C, et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ Open 2013;3: e003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Org E, Veldre G, Viigimaa M, et al. HYPEST study: profile of hypertensive patients in Estonia. BMC Cardiovasc Disord 2011; 11: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Babor T, Caetano R, Casswell S, et al. Alcohol: No Ordinary Commodity. Research and Public Policy. Oxford: Oxford University Press, 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.