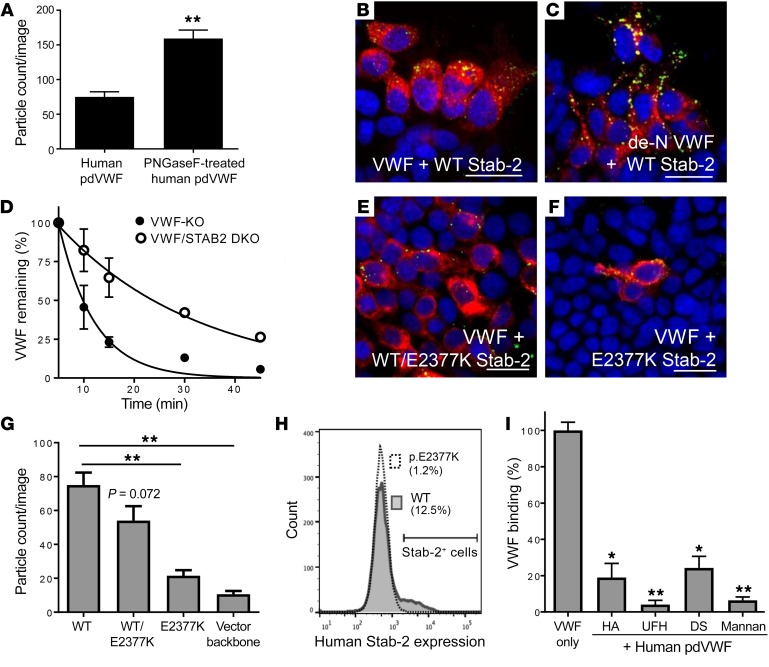
Figure 6. Genetic and biochemical regulation of VWF interactions with stabilin-2.
HEK 293 cells were transiently transfected with the human stabilin-2 cDNA and incubated with 2 U/ml human pdVWF or 2 U/ml human pdVWF pretreated with PNGase F to remove its N-linked glycans (de-N VWF). (A) Quantification of de-N VWF binding to stabilin-2–expressing cells. (B and C) Human pdVWF and de–N-glycosylated VWF (green) binding to human stabilin-2–expressing (red) cells. Images are representative of n = 6 independent experiments. Scale bars: 40 μm. (D) The influence of VWF N-linked glycans on the clearance of VWF by stabilin-2 was characterized in vivo (n = 8 animals per condition). See statistical summary of half-life studies in Table 1. The stabilin-2 variant p.E2377K was introduced into the human stabilin-2 cDNA by site-directed mutagenesis. (E and F) HEK 293 cells were transiently transfected with the stabilin-2 cDNA p.E2377K 1:1 with WT (E, red) or p.E2377K stabilin-2 alone (F, red) and incubated with 2 U/ml human pdVWF (green). Images are representative of n = 4 independent experiments; scale bars: 40 μm. For all experiments, blue indicates DAPI; and yellow, colocalization. (G) Quantification of VWF binding to HEK 293 cells expressing the p.E2377K stabilin-2 variant. (H) Flow cytometric analysis of p.E2377K stabilin-2 expression. Pathogenicity assessment of the stabilin-2 p.E2377K variant is described in Table 2. To demonstrate competitive binding to stabilin-2, known stabilin-2 ligands were preincubated with HEK 293 cells expressing murine stabilin-2 for 15 minutes and then incubated with 2 U/ml human pdVWF for 1 hour. (I) Quantitative IF was performed to compare untreated VWF binding to cells pretreated with stabilin-2 ligands (n = 3–4 independent experiments). HA, hyaluronic acid; DS, dermatan sulfate; UFH, unfractionated heparin. Throughout figure, *P < 0.05, **P < 0.001 as determined by t test.