Abstract
Purpose:
We investigated the relationship between visual parameters that are commonly affected during glaucomatous disease progression with functional measures of retina physiology using electroretinography and behavioral measures of visual function in a mouse model of glaucoma. Electroretinogram components measuring retinal ganglion cell (RGC) responses were determined using the non-invasive Ganzfeld flash electroretinography (fERG) in order to assess RGC loss in a mouse model of glaucoma.
Methods:
Intraocular pressure (IOP), behaviorally assessed measures of visual function, namely visual acuity and contrast sensitivity as well as fERG responses were recorded in four-month and 11-month old male DBA/2 mice. Scotopic threshold response (STR) and photopic negative response (PhNR) components as well as oscillatory potentials (OPs) were isolated from fERG responses and correlated with IOP, optomotor reflex measurements and RGC counts.
Results:
11-month old DBA/2 mice had significantly elevated IOP, reduced visual performance, as assessed behaviorally, significant RGC loss, deficits in standardized fERG responses, reduced STRs, and differences in OP amplitudes and latencies, when compared to four-month old mice of the same strain. STRs and OPs correlated with some visual and physiological parameters. In addition, elevated IOP and RGC loss correlated positively with measures of visual function, specifically with surrogate measures of RGC function derived from fERG.
Conclusion:
Our data suggest that RGC function as well as interactions of RGCs with other retinal cell types is impaired during glaucoma. In addition, a later oscillatory potential wavelet denoted as OP4 in this study was identified as a very reproducible indicator of loss of visual function in the glaucoma mouse model.
Keywords: Glaucoma, visual acuity, contrast sensitivity threshold, intraocular pressure, electroretinography, retinal ganglion cells, scotopic threshold response, oscillatory potentials
Introduction
Using rodent models of disease presents a clear advantage in studying visual function due to their low-cost and maintenance, well-defined anatomy and physiology, short lifespan, and the variety of transgenic models available.1 A common method to assess visual function in rodents is to use the optomotor reflex (OMR), in order to determine visual acuity and contrast sensitivity thresholds.1 This method is highly reliant on functioning RGCs and generally considered to involve the whole visual pathway or most of its components, although several mechanisms underlying the optomotor response still need to be fully characterized.1 A common assessment of retinal function in rodents is Ganzfeld flash electroretinography (fERG), a non-invasive, non-subjective, method measuring full-field retinal potentials in response to light stimuli, where components of the fERG response represent the activity of specific cell populations in the retina.2–4 An fERG component, the scotopic threshold response (STR), is thought to be an indicator of RGC and amacrine cell function in the proximal retina.5 This is measured at very low light intensities that can be easily saturated, which is a drawback to using this component. Previous studies have reported that the STR is saturated above light intensities as low as −6.3–6.5 log cd·s/m2 for rats and mice6 whereas other studies report higher intensities such as −3.6 log cd·s/m2 7 are adequate for mice and −4 log cd s/m2 for mice3 and rats2. Based on these reports, we determined that STRs measured from light intensities at −4.5 and −4 log cd s/m2 would be most relevant to the current study that used a mouse model of glaucoma. Another potential measure of RGC function using fERGs are oscillatory potentials (OPs), which are wavelets on the ascending portion of the b-wave of the fERG trace and presumably measure inner retinal function including RGCs.8–10
The development of pigmentary glaucomatous optic neuropathy in the DBA/2 mouse model resulted from spontaneous mutations and characterized by chronic retinal neurodegeneration increasing in severity with age. It has several similarities to glaucoma in human patients including loss of vision and RGC loss, as has been described before by others and us and as is also the case for other animal models of glaucoma1, 11–16. Several studies have demonstrated that IOP increases with age in DBA/2 strains (starting between 8–12 months) which is not significantly altered in ageing control, wild-type C57BL/6 mice (ages range from 2–15 months).16–19 Visual acuity in DBA/2 mice (using the optomotor reflex) was shown to be similar between DBA/2 and C57BL/6 mice from 1–5 months of age, but started to decline in DBA/2 mice at 7 months and was severely affected by 12–14 months of age, whereas visual acuity in C57BL/6 mice remained unchanged with increasing age.20 It has been previously reported that ageing DBA/2 mice exhibit significant changes in fERG parameters similar to those that we are using here, such as reduced a-wave or b-wave amplitudes21, 22, STRs16, 17 and OPs21, whereas these parameters were either unaltered or exhibited a slight, non-significant decrease with age when measured in aging C57BL/6 controls in the same studies. Similarly, scotopic a- and b-wave amplitudes in three-month old DBA/2 mice had no abnormalities and are comparable to age-matched C57BL/6 mice19, 23, whereas in two year old DBA/2 mice both scotopic a- and b-wave amplitudes and RGC numbers were reduced significantly compared to age-matched C57BL/6 controls.24 Based on these published findings, we reason that normal age-related changes have had no significant impact on the data measured in this study using similar experimental parameters. Additionally, these reports confirm that four-month old DBA/2 mice are non-glaucomatous and similar to “normally aged” wildtype controls, whereas 11-month old DBA/2 mice exhibit deterioration of visual parameters related to glaucoma pathology and were therefore chosen for control and experimental groups, respectively.
Here, we present evidence for a strong relationship between visual parameters commonly affected during glaucoma disease progression and select components of Ganzfeld fERGs representing RGC function, specifically STRs and OPs. Our goal was to employ indicators of RGC function derived from non-invasive fERG in order to quantify RGC loss and loss of function in glaucomatous retinopathy using a common mouse model of glaucoma.
Methods
Animals
Four-month old (n=5 mice) and 11-month old (n=5 mice), male DBA/2 mice were obtained from the National Institute on Aging (NIA) Aged Rodent Colonies (Wilmington, MA). All animals were socially housed, five mice per cage, and had ad libitum access to food and water while being maintained on a 12-hour light/dark cycle. All animal husbandry and experimental procedures were conducted in compliance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, in accordance with the ARVO Animal Statement and with institutional guidelines and had been approved by the Institutional Animal Care and Use Committee.
Optomotor reflex measurements
Visual acuity (VA) and contrast sensitivity thresholds (CST); together known as visuospatial measurements, were determined using the OptoMotry© optokinetic testing system (CerebralMechanics, Lethbride, AB, Canada) as described previously 25-28. We employed this assessment of the reflexive optomotor response (OMR) or “optocollic” response29 by placing an animal on to a platform where stimuli with patterns of varying spatial frequencies (cycle/degree; c/d) or contrast levels (%) were randomly projected until head tracking (optomotor responses) was no longer detected; thereby establishing VA and CST levels, respectively. By changing the direction of the stimulus pattern left or right eyes can be tested individually due to the asymmetry of the optomotor response, 26 therefore thresholds determined from individual eyes were considered individually for statistical analyses, i.e. each eye representing n=1. The VA threshold was determined with the contrast set at 100% and the CST was determined with the spatial frequency set at 0.042 c/d 25–28.
Intraocular pressure measurements
Intraocular pressure was measured in fully conscious mice using a rebound tonometer (Icare TONOLAB, Colonial Medical Supply Co. Inc., Franconia, NH). The data was expressed as an average of three IOP readings per eye (each eye representing n=1), with one reading consisting of six rapid tonometer measurements averaged and only accepted if the standard deviation is normal.
Ganzfeld flash electroretinography
Ganzfeld fERG procedures in mice were performed under dim red light following overnight dark adaptation (>12 hours). Mice were anesthetized using isoflurane at 3% to induce and 1.5% to maintain anesthesia. The pupils were dilated with 1 drop of 1% tropicamide and allowed to dilate for 10 minutes. Rectal temperature was monitored and maintained at 37°C with a heating pad. Silver-embedded thread electrodes were placed over the cornea in 1% methylcellulose with mini-contact lenses fitted in order to prevent corneal dehydration (Ocuscience LLC, Henderson, NV). The head was positioned inside the Ganzfeld dome, and fERG with two recording channels was performed using an HMsERG system (Ocuscience LLC) that has an amplifier with a band pass from 0.3Hz to 300Hz. Mice were subjected to the International Society for Clinical Electrophysiology of Vision (ISCEV) standardized ERG protocol30, which was implemented as follows: overnight dark adaption (>12 hours) (1) −2.0 log cd·s/m2 (rod response; 10 flashes); (2) 0.5 log cd·s/m2 (standard rod–cone response; 4 flashes); (3) 1.0 log cd·s/m2 (Hi-intensity rod–cone response; 4 flashes); light-adaption (10 minute) with background illumination (1.5 log cd·s/m2) (4) 0.5 log cd·s/m2 (cone response; 32 flashes); (5) 1.0 log cd·s/m2 (Hi-intensity cone response; 32 flashes); (6) 0.5 log cd·s/m2 flicker at 30 Hz (standard; 128 flashes) (7) 1.0 log cd·s/m2 flicker at 30 Hz (Hi-intensity; 128 flashes). ERGView 4.380V software (OcuScience LLC) was used to average multiple flashes recorded at each intensity and stored for further analysis.
Mice were also tested using a scotopic flash intensity series (−4.5 – 1.5 log cd·s/m2), where a 1:1,000 neutral density filter (ND3) was used to control the seven lowest flash intensities; data was averaged from 10 flashes (−4.5 – −3.5 log cd·s/m2), 4 flashes (−3 – 0.5 log cd·s/m2) at the lower intensities or measured from 1 flash at the two highest intensities (1 – 1.5 log cd·s/m2). Following light adaptation (1.5 log cd·s/m2 for 10 minutes), responses from a photopic series (−2 – 1.5 log cd·s/m2; 32 flashes per intensity) were recorded separately.
fERG Analysis
All a- and b-wave amplitudes and implicit times were obtained from ISCEV and scotopic fERG intensity series responses (without filtering) using ERGView 4.380V software, while photopic fERG intensity series responses were filtered using a 150Hz low-pass filter. The a-wave amplitude was determined as the distance from the baseline to the trough of the a-wave, whereas the amplitude of the b-wave was calculated as the distance from the trough of the a-wave to the peak of the b-wave. The implicit time was considered as the time needed to reach the trough of the a-wave or the peak of the b-wave. Averaged responses from scotopic and photopic intensity series were plotted using GraphPad Prism 5 software and non-linear regression analysis was performed using the following hyperbolic function (stimulus-response):
The stimulus-response function is widely used to represent the relationship between rod a- and b-wave responses and flash intensity31, where I=flash intensity (cd·s/m2); V=response at I; n=slope; Vmax=maximal response and σ=the flash intensity at which half the maximal response is achieved.
STRs were measured from traces produced by the fERG scotopic intensity series and considered to be generated at flashes below −3.6 log cd·s/m2, as defined in Smith et al., 2014 7. The positive STR (pSTR) was considered to be the maximum voltage change above baseline32 between 10-200ms33, and the negative STR (nSTR) was the minimum change in voltage below baseline between 150–250ms after the flash33 (Fig. 5A). PhNR was considered the local minimum response 100–250ms after the flash.
Figure 5:
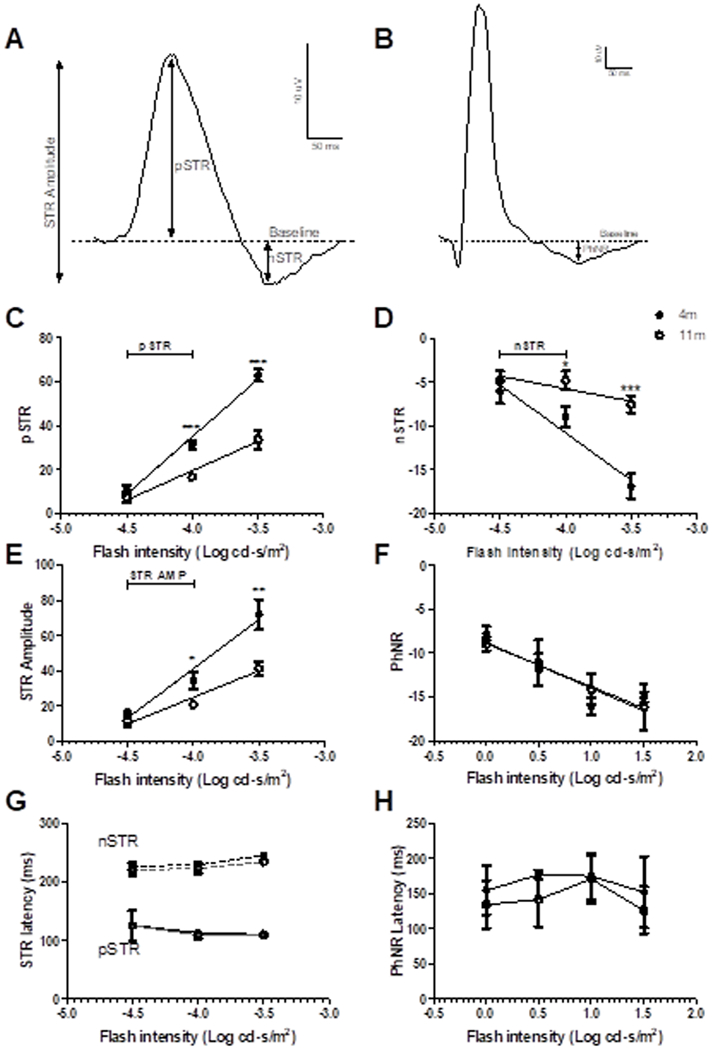
pSTR (A, C), nSTR (A, D), STR amplitudes (A, E) and PhNR (B, F) were measured from responses to flashes below −4.5 - −3.5 log cd·s/m2 for scotopic and 0–1.5 log cd·s/m2 for photopic responses of 4 month-old (closed circles) and 11-month old (open circles) DBA/2 mice. In responses to flash stimuli below −3.6 log cd·s/m2, where STRs indicate RGC function, 11-month old DBA/2 mice had significantly reduced STRs at −4 log cd·s/m2 when compared to 4 month-old DBA/2s. The latency of the STRs (G) and PhNR (H) was not significantly different between ages. Data are expressed as mean ± SEM.
The OPs were extracted from fERG responses generated from flashes equal to and higher than 0.5 log cd·s/m2 (OP1) or −2 log cd·s/m2 (OP2-4) by digital filtering of the raw signal with the bandwidth of 60–300Hz using ERGView 4.380V software, where XY values were plotted in GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA) and “XY analysis”, “Area Under the Curve” analysis function was used to find the amplitude and latency of the relevant peaks (OP1–4, Fig. 6A and B). The “Area Under the Curve” analysis function returns the Y value, i.e. amplitude, and X value, i.e. time, of each peak maximum on the plotted curve. Therefore the X and Y values for each relevant peak were generated from this analysis and were used to determine the OP1–4 data (Fig. 6), the area under the curve values were not considered in this study.
Figure 6:
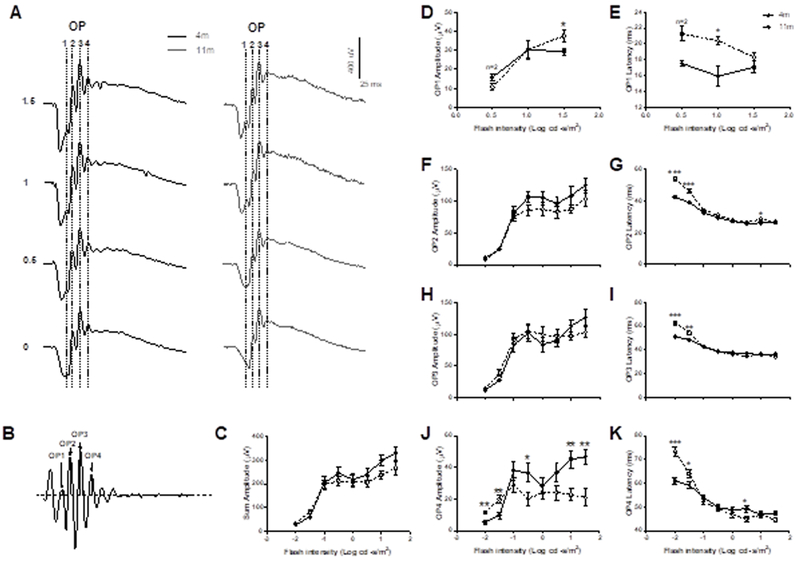
Scotopic oscillatory potential amplitudes, a measure of RGC function, were analyzed with regard to latency and amplitude at 4 peaks / local maxima termed OP1–4 (A, B) and were measured from the baseline (black dashed horizontal line) to the local maximal of the positive peak (grey dotted vertical line; B). There was no significant difference in OP1–4 amplitudes between the two age groups (C), however, 11-month old DBA/2 mice showed increased amplitudes at OP1 (D), unchanged amplitudes at OP2 (F) and OP3 (H) and decreased amplitudes at OP4 (J). Latencies were slower at OP1 (E) and at lower flash intensities for OP2 (G), OP3 (I) and OP4 (K). Data are expressed as mean ± SEM.
Numbers from ISCEV, STR and OP measurements that fell 1.5 times the interquartile range outside the upper or lower interquartile range was excluded from the mean, in order to eliminate outliers that were caused by, for example, excessive noise interference.
Antibodies
Proteins serving as retinal cell markers identifying distinct populations of cells were used to determine significant changes in overall structural integrity of the retina comparing four-month and 11-month old DBA/2 retinae. Rhodopsin (Rho; Chemicon catalog number: MAB5256; MilliporeSigma, Burlington, MA) was used to label rod outer segment. Glycogen phosphorylase (GP)34 was used to label cone photoreceptors in the outer nuclear layer (ONL) and GP-positive bipolar cells in the inner nuclear layer (INL) (1∶1000; guinea pig polyclonal antibody; generous gift from Dr. B. Hamprecht, University of Tübingen, Tübingen, Germany35, 36). Protein kinase C alpha (PKCα; rabbit polyclonal; Sigma catalog number, P4334; MilliporeSigma) was used as a well-established rod bipolar cell marker37, and Brn3a as an RGC marker38 (Millipore catalog number, MAB1585; MilliporeSigma).
Immunohistochemistry
One retina from each mouse was fixed, cryo-sectioned and immunostained as described before9. The cryosections were immunostained with rabbit anti- PKCα (1:10,000), guinea pig anti-GP (1:2000), mouse anti-Brn3A (1:150) or mouse anti-Rho (1:1000) and fluorescently labeled (Alexa Fluor goat anti-guinea pig 488; 2μg/ml; Invitrogen, A-11073; Alexa Fluor goat anti-mouse 555; 2μg/ml; Invitrogen, A-21424; Alexa Fluor goat anti-rabbit 633; 2μg/ml; Invitrogen, A-21071 and nuclei were stained histologically with 4’,6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI; MilliporeSigma) as described before9.
Quantification of retinal thickness, cell counts and immunoreactivity
Retina sections were imaged using a Nikon C2+ confocal imaging system (Nikon, New York, NY). Cell counts, retinal thickness and fluorescence intensity (for Rhodopsin immunoreactivity) were collected in a 500µm retinal length by a masked observer on a maximum projection image of the retina (n=5 retinas per age group) approximately 800–1000µm from the optic nerve head. Cell numbers and retinal thicknesses were adapted from a quantification paradigm described in Chrysostomou et al. (2013).4 Using the “Cell Counter” plug-in in Fiji image analysis software,39 cone photoreceptors, bipolar cells, rod bipolar cells and retinal ganglion cells were counted and expressed as number of cells per mm retinal length.4 Retinal thickness for ONL, OPL, INL, IPL and overall retina was measured on DAPI-stained cryosections (n=5 per age group) using ImageJ / Fiji image analysis software39. Levels of rhodopsin immunoreactivity were also measured with ImageJ / Fiji image analysis software39, and the average of 10 regions of interest (ROIs) with a surface area of 200µm2 placed along the outer segment of the retina (n=5 retinas per age group) was calculated for statistical analysis.
Statistical Methods
All fERG data (expressed as mean ± SEM) were plotted and statistically analyzed in GraphPad Prism 5. For each parameter, Student’s t-tests were performed for comparisons between four-month-old and 11-month old DBA/2 mice on fERG data collected using the ISCEV standardized ERG protocol. For scotopic and photopic intensity series data, a two-way ANOVA with Bonferroni correction for post hoc analysis was performed between four-month old and 11-month old DBA/2. For statistical analysis of Vmax, σ and n, amplitudes of individual eyes from the scotopic and photopic intensity series were plotted and analyzed using the stimulus-response function, where data that did not fit this function were returned by the statistical function as ambiguous or interrupted results and were not used in the final analysis.
For correlation analyses, the Pearson product-moment correlation coefficient (r) was determined 40, 41, and correlational strength defined as, no correlation for 0.0 ≤ r ≤ 0.2, weak correlation for 0.2 ≤ r ≤ 0.4, moderate correlation for 0.4 ≤ r ≤ 0.6, and strong correlation for 0.6 ≤ r ≤ 1.0 as described previously 40, 41. Significant correlations are labelled with both the Pearson product-moment correlation coefficient r and p-values (Figs. 7 and 8).
Figure 7:
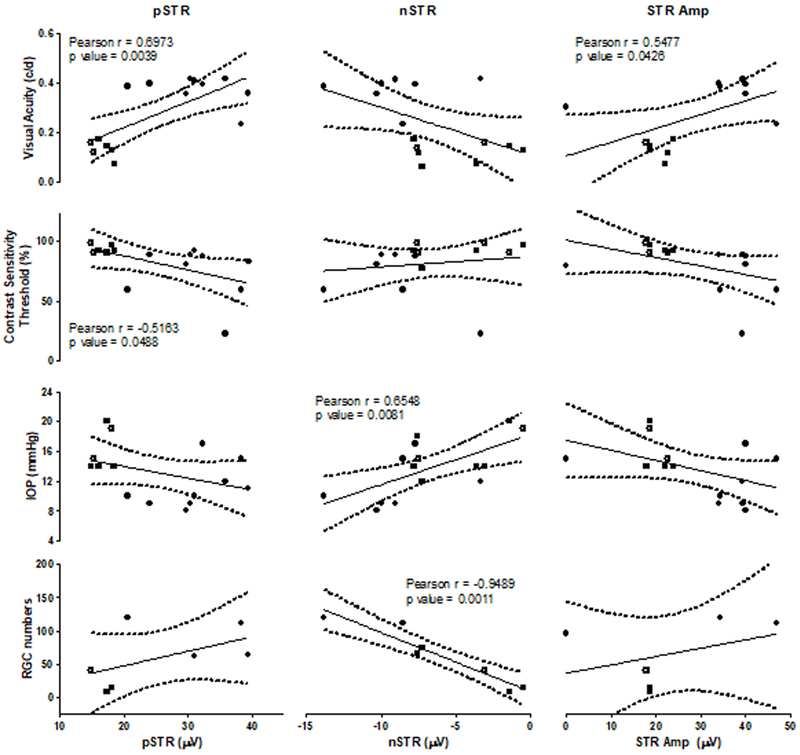
STRs recorded at a flash intensity of −4 log cd·s/m2 were correlated with Visual Acuity, Contrast Sensitivity Threshold, IOP and RGC counts, respectively. Specific Pearson product-moment correlation coefficient r and respective p-values are listed directly in panels, where significant correlations were identified.
Figure 8:
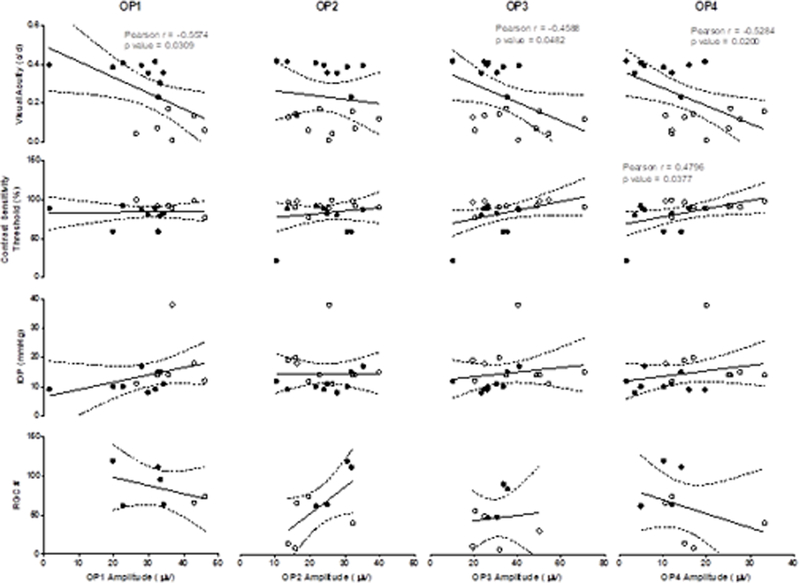
OP amplitudes recorded at flash intensities of 1.5 (OP1) or −1.5 log cd·s/m2 (OP2–4) were correlated with Visual Acuity, Contrast Sensitivity Threshold, IOP and RGC number. Specific Pearson product-moment correlation coefficient r and respective p-values are listed directly in panels, where significant correlations were identified.
Results
11-month old DBA/2 mice display critical hallmarks of glaucoma
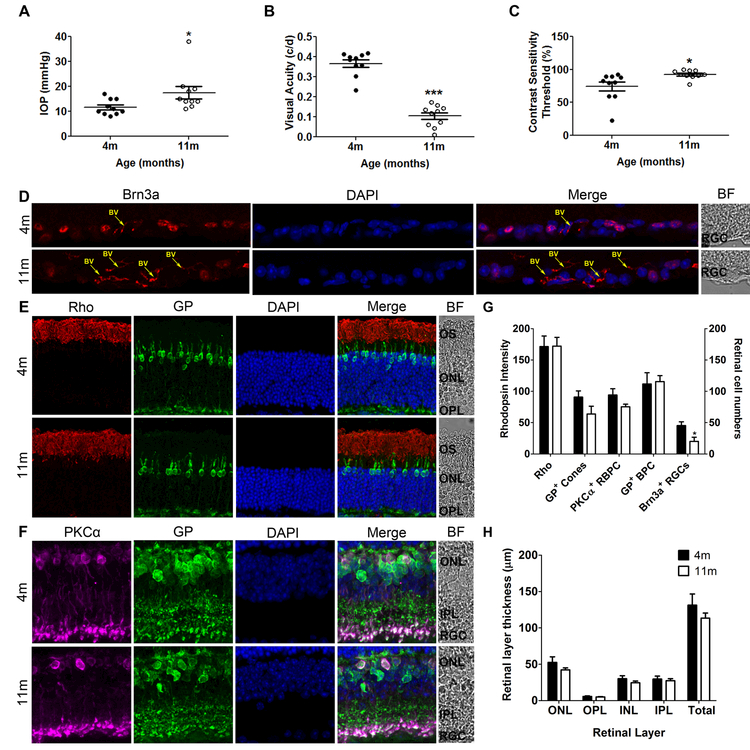
11-month old DBA/2 mice had significantly increased IOP (17.5 ± 2.46mm Hg, p<0.05), CST (92.57 ± 2.10%, p<0.05) and significantly decreased VA (0.1045 ± 0.0170 c/d, p<0.001) and RGCs (20.2 ± 6.6 cells/mm, p<0.05) compared to four-month old IOP (11.6 ± 1.0mm Hg, Fig. 1A), CST (74.2 ± 6.8%, Fig. 1C), VA (0.3666 ± 0.0185 c/d, Fig.1B) and RGC (45.4 ± 6.0 cells/mm; Fig.1D, G). There were no significant differences in other retinal cell layers (Fig. 1E-H).
Figure 1:
Eleven-month old DBA/2 mice have increased IOP (A), reduced visual function [measured as reduced visual acuity (B) and increased contrast sensitivity threshold (C)] and significant RGC loss (Brn3a-positive cells in D [red label], G) indicative of glaucoma disease progression and when compared to young, non-glaucomatous control mice. Photoreceptor (E, G) and bipolar cell numbers (F, G) and retinal layer thickness (H) in 11-month old glaucomatous mice are not significantly different from 4 month-old non-glaucomatous mice; data are expressed as the number of cells per 1-mm retinal length in a vertical section or the thickness of the retina or layer in µm. In E and F, rhodopsin (Rho) immunoreactivity was found in the outer segments of the rod photoreceptors (red); immunoreactivity for Glycogen Phosphorylase (GP) labelled cone photoreceptors, cone bipolar cells and their terminals (green), and for Protein Kinase α (PKCα) labelled rod bipolar cells and their terminals (purple) for Brn3a labelled RGCs. Nuclei were stained with DAPI (blue). BV= Blood vessel. Data are expressed as mean ± SEM.
11-month old DBA/2 mice show deficits in ISCEV fERG function
ISCEV ERG tests (Fig. 2A-C, 3A-E) showed that 11-month old DBA/2 mice had a significantly longer a-wave implicit time (Fig. 3A) for standard rod-cone (17.61 ± 1.0ms, p< 0.0001), hi rod-cone (14.06 ± 0.30ms, p< 0.0001), and cone (27.16 ± 1.80ms, p<0.05) ISCEV tests compared to four-month old standard rod-cone (12.8 ± 0.1ms), hi rod-cone (11.33 ± 0.18ms) and cone (19.46 ± 1.99ms) responses, and longer b-wave implicit (Fig. 3B) time for rod responses (69.86 ± 2.33ms, p< 0.0001) compared to four-month old mice (54.79 ± 1.24ms). Older DBA/2 mice had lower a-wave amplitudes (Fig. 3C) for rod (12.12 ± 2.49µV, p<0.05) and hi rod-cone (322.2 ± 16.3µV, p<0.05) ISCEV ERG tests compared to four-month old DBA/2 rod (19.39 ± 2.02µV) and hi rod-cone (371.1 ± 9.0µV), and lower b-wave amplitudes (Fig. 3D) for rod (235.7 ± 17.8 µV, p<0.01) and cone (69.21 ± 5.10µV, p<0.05) ISCEV ERG responses compared to four-month old rod (297.6 ± 7.4µV) and cone (86.7 ± 4.8µV) responses.
Figure 2:
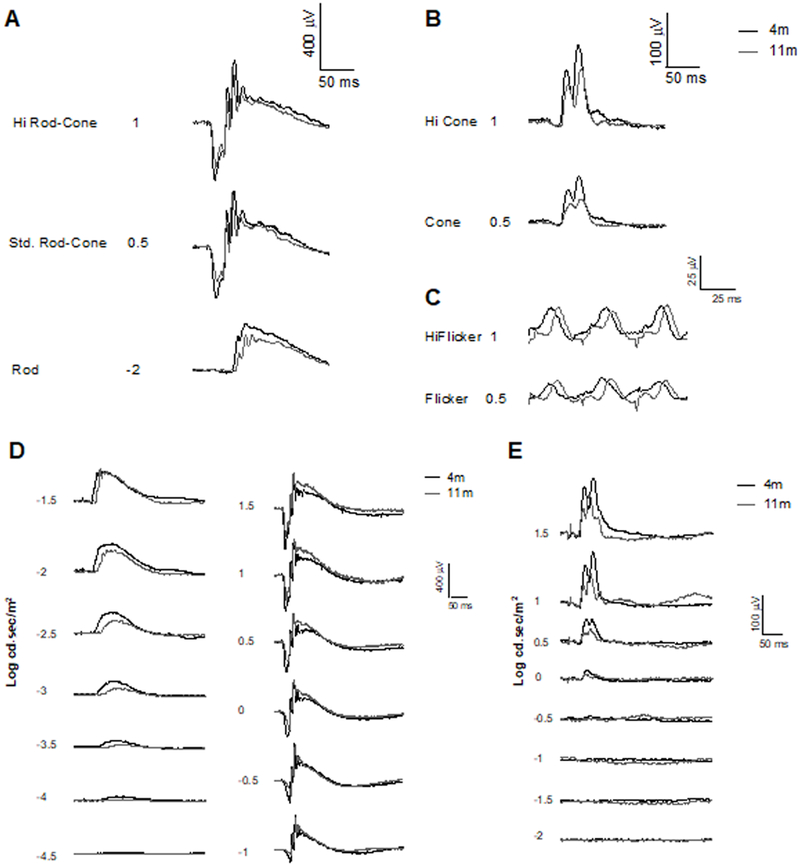
Example traces from A) rod, standard rod–cone, and Hi-intensity rod–cone ISCEV (1–3) protocol response. Following light-adaption (10 minute) with background illumination B) cone and Hi-intensity cone ISCEV (4–5) protocol response and C) standard and Hi-intensity flicker flicker at 30 Hz ISCEV (6–7) protocol response. D) Scotopic and E) Photopic fERG intensity series in 4 month-old (black line) and 11-month old (gray line) DBA/2 mice. Flash intensities are expressed in log cd·s/m2.
Figure 3:
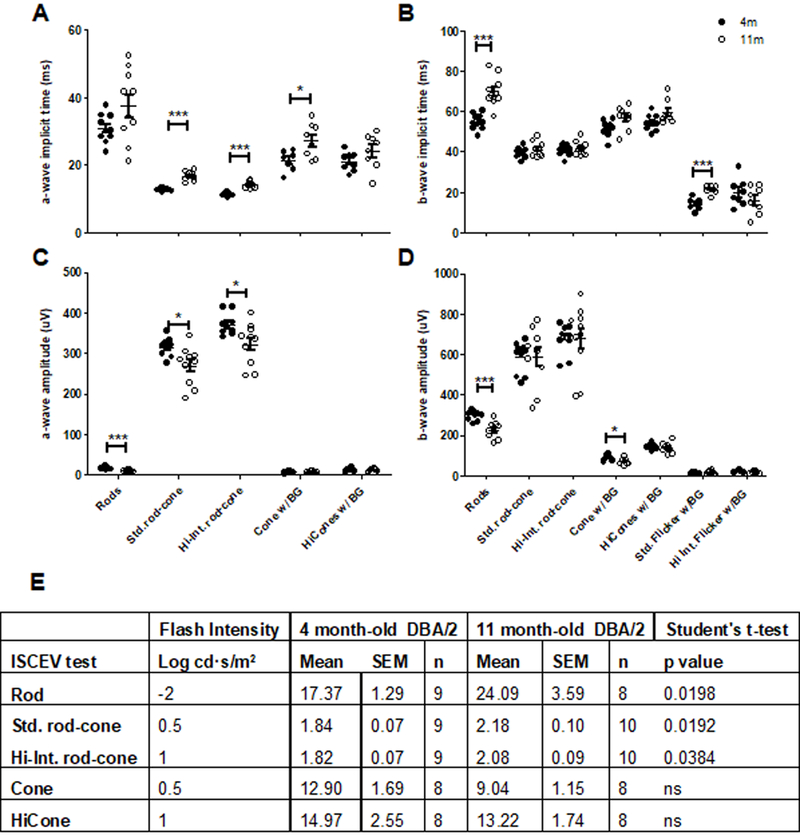
Implicit times of a-waves (A) and b-waves (B) and their respective amplitudes (C, D) and the subsequent b/a ratio of the a- and b-wave amplitudes (E) were determined for 4 month-old (closed circles) and 11-month old (open circles) DBA/2 mice.
11-month old DBA/2 mice had significantly higher b/a ratios (Fig. 3E) for standard rod-cone (2.176 ± 0.104, p<0.05) and hi rod-cone (2.078 ± 0.091, p<0.05) compared to four-month old mice (1.878 ± 0.071 and 1.816 ± 0.071, respectively).
11-month old DBA/2 mice show deficits in fERG responses to varying flash intensities
Scotopic amplitudes (Fig. 4A) for the fERG flash intensity series (Fig. 2D, 4A, C, and E) were significantly decreased in 11-month old a-waves (−1 – 1.5 log cd·s/m2, p<0.001) and b-waves (−2.5—2, −0.5, 0.5–1.5 log cd·s/m2, p<0.05–0.0001) flash intensities compared to four-month old DBA/2 mice. Stimulus-response non-linear regression analysis show that maximal response (Vmax) parameters are significantly lower in 11-month old scotopic a-wave (263.8 ± 21.3µV, p<0.0001) and b-wave (606.6 ± 29.5µV, p<0.01) amplitudes compared to four-month old mice (501.8 ± 8.8µV and 916.9 ± 87.2µV, respectively). Eleven month old mice had a significantly steeper slope (n) for scotopic a-wave (0.99 ± 0.07) and b-wave (0.55 ± 0.02) compared to four-month old mice (0.65 ± 0.03 and 0.40 ± 0.04, respectively). Scotopic a- and b-wave implicit times remained largely unchanged, except for the a-wave implicit time at −2 log cd·s/m2.
Figure 4:
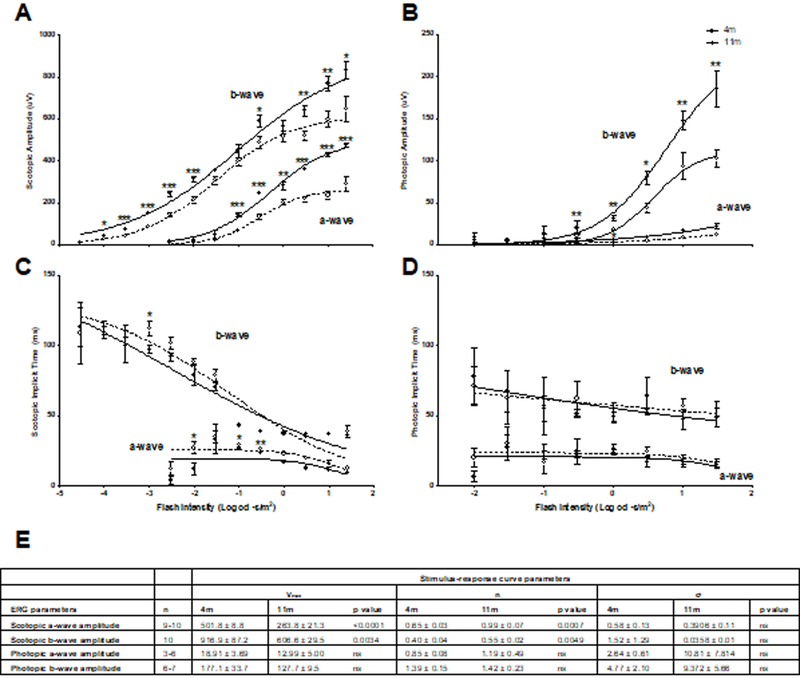
11-month old glaucomatous (open circles) DBA/2 mice show deficits in scotopic (A) and photopic amplitudes (B) compared to young, 4 month-old non- glaucomatous mice (closed circles), however, the scotopic (C) and photopic (D) a- and b-wave implicit times remained largely unchanged. Data are expressed as mean ± SEM.
Photopic amplitudes (Fig. 4B) were significantly decreased in 11-month old a-wave (1.5 log cd·s/m2, p<0.05) and b-waves (0.5 – 1.5 log cd·s/m2, p<0.05 – 0.001), however implicit times were unchanged (Fig. 4D). Stimulus-response non-linear regression analysis indicated that the Rmax, n and sigma parameters for scotopic implicit time, and photopic amplitudes and implicit time, were not significantly different.
11-month old DBA/2 mice showed a decrease in fERG measures of RGC function
11-month old DBA/2 mice had significantly lower pSTRs (16.71 ± 0.62µV, p< 0.0001) nSTRs (−4.836 ± 1.075µV, p<0.05) and STR Amp (20.68 ± 1.03µV, p<0.05) at flash intensity −4 log cd·s/m2, compared to four-month old pSTR (31.3 ± 2.068µV), nSTR (−8.958 ± 1.194 µV) and STR Amp (34.25 ± 5.09, µV), but no significant differences between ages were found at flash intensity −4.5 log cd·s/m2. There was no difference between latencies of pSTR (109.5 ± 5.8ms) and nSTRs (223.1 ± 9.6ms) in older mice compared to younger mice (113.3 ± 3.0ms, and 229 ± 6ms, respectively). Although not considered a true STR in this study, 11-month old mice had significantly lower pSTRs (33.63 ± 4.25µV, p< 0.0001) nSTRs (−7.542 ± 0.980µV, p< 0.0001) and STR Amp (41.17µV ± 4.01, p<0.01) at flash intensity −3.5 log cd·s/m2 compared to four-month old pSTR (62.89 ± 2.70µV) nSTR (−16.88 ± 1.40µV) and STR Amp (71.79 ± 8.64µV). PhNR and PhNR latencies are not significantly different between four-month and 11-month old mice at any flash intensity.
11-month old mice showed differences in latency and amplitude of oscillatory potentials
OP1 was generated at a flash intensity of 0.5 log cd·s/m2 and higher in four-month old DBA/2 mice, whereas only one 11-month old mouse (both eyes, n=2) generated an OP1 wavelet at this intensity (noted in Fig. 6D), however they were detected in both ages at flash intensity 1 log cd·s/m2 and higher (Fig. 6A, D). The OP1 amplitude (Fig. 6D) was unchanged at 1 log cd·s/m2 flash intensity, however, its latency (Fig. 6E) was significantly longer in older mice (20.4 ± 0.5ms, p<0.05) compared to younger mice (15.96 ± 1.28ms). The OP1 amplitude was significantly larger in 11-month old mice (37.61 ± 3.40µV, p<0.05) at flash intensity 1.5 log cd·s/m2 compared to four-month old mice (29.43 ± 1.69µV), however the latency at this flash strength was unchanged. OP2 amplitudes (Fig. 6F) were not significantly different at any flash strength, however the latency (Fig. 6G) was longer in older mice at −2 (53.72 ± 1.48ms, p< 0.0001) and −1.5 (46.11 ± 1.30ms, p<0.001) log cd·s/m2 compared to four-month old mice (42.49 ± 0.79 and 38.93 ± 0.68, respectively). OP3 amplitudes (Fig. 6H) were not significantly different at any flash intensity, however the latency (Fig. 6I) was longer in older mice at −2 (62.87 ± 1.75ms, p<0.0001) and −1.5 (54.55 ± 1.56ms, p<0.01) log cd·s/m2 compared to four-month old mice (51.28 ± 0.93ms and 48.5 ± 0.9ms, respectively). OP4 amplitudes (Fig. 6J) were significantly larger (p<0.01) in older mice at lower intensity flashes (−2 and −1.5 log cd·s/m2) but smaller (p<0.05–0.01) at higher intensity flashes (−0.5, 1 and 1.5 log cd·s/m2). OP4 latency (Fig. 6K) was longer in older mice at lower intensity flashes, −2 (73.19 ± 2.00ms, p<0.0001) and −1.5 (63.99 ± 1.77ms, p<0.05, n=10) log cd·s/m2, compared to four-month old mice (60.91 ± 1.34ms and 59.24 ± 1.21ms, respectively), but was incrementally faster at the higher flash intensity of 0.5 log cd·s/m2 (44.94 ± 1.24ms, p<0.05) when compared to younger mice (49.28 ± 1.31ms).
Behavioral measures of visual performance correlate with fERG measures of RGC function
STRs were significantly correlated with behavioral measures of visual performance, IOP and RGC counts (Figure 7). pSTRs were significantly and positively correlated with visual acuity (p<0.01), while they were significantly and negatively correlated with the contrast sensitivity threshold (p<0.05), i.e. reduced visual performance was correlated with lower pSTRs. nSTRs were significantly and negatively correlated with IOP (p<0.01), i.e. higher levels of the IOP, indicative of potentially greater visual impairment resulting from glaucoma, were correlated with lower, more positive nSTR values. RGC numbers were significantly and positively correlated with nSTR (p<0.01), i.e. lower RGC counts, again indicative of a more severe glaucoma phenotype were associated with lower, more positive nSTR values. STR amplitudes were significantly and positively correlated with visual acuity (p<0.05).
OP amplitudes were significantly correlated with behavioral measures of visual performance, IOP and RGC counts (Figure 8). Amplitudes of OP1, OP3 and OP4 were significantly (p<0.05) and positively correlated with visual acuity, while only the OP4 amplitude was significantly (p<0.05) and negatively correlated with the contrast sensitivity threshold (p<0.05), i.e. reduced visual performance was correlated with lower the amplitudes of OPs. Similarly, increased OP latencies were significantly correlated with reduced visual performance and RGC numbers as well as elevated IOP (data not shown).
Discussion
Here, we investigated the relationship between parameters of retina function and visual performance that are commonly affected during glaucomatous retinopathy progression. Our goal was to determine surrogate markers of RGC function taken from fERG recordings, specifically the STRs and OPs, in order to quantify RGC loss and loss of function with non-invasive fERG during disease progression in a widely used mouse model of glaucoma. It is important to note that the anesthetic used during fERG for all mice in our study was isoflurane, and although isoflurane has been widely used for rodent ERG protocols and has been previously recommended as an alternative to ketamine anesthetic for the mouse ERG42. Isoflurane has also been shown to reduce ERG responses in rats when compared to other forms of anesthetic such as ketamine/xylazine43, and also appears to increase an early OP wavelet.44 Woodward et al. (2007) suggested for mouse ERG that “statistical comparisons be limited to studies using the same form of anesthesia”, refering to the use of isoflurane versus ketamine/xylazine.42 Accordingly, all mice in our study were subjected to the same anesthetic procedure, which allowed us to apply adequate statistical comparisons.
As described previously by others and us, the DBA/2 mouse model of pigmentary glaucoma develops chronic age-related retinal neurodegeneration and exhibits multiple similarities to the human disease condition.1, 11–15, 25 11-month old DBA/2 mice exhibited 1) typical signs of glaucomatous retinopathy with high IOP measurements, reduced visuospatial function and significant RGC loss; 2) deficits in rod, mixed rod/cone and cone responses in standardized ISCEV fERG protocols; 3) deficits in a- and b-wave amplitudes; 4) reduced STRs; and 5) differences in OP amplitudes and latencies, when compared to non-glaucomatous, four-month old DBA/2 mice. STRs and OP amplitudes and latencies isolated from fERGs as measures of RGC responses were significantly correlated with visual and physiological parameters commonly affected in glaucoma disease progression and could potentially serve as surrogate markers of disease.
Evidence for glaucomatous retinopathy in the 11-month old DBA/2 mouse cohort
Older, i.e. 11-month old mice were considered the experimental cohort (glaucomatous) and younger, i.e. four-month old mice as the control cohort (non-glaucomatous). We found that 11-month old DBA/2 mice had increased IOP (Fig. 1A), decreased visual function (Fig. B, C) and a significant reduction in RGC numbers (Fig. 1D, G), compared to four-month old mice, typical of a glaucomatous phenotype. We also observed a trend towards thinning of retinal layers that did not reach statistical significance (Fig. 1H), but this trend is likely to continue with disease progression. Age-related increases in IOP 12, 13, 15–19, 21, 45, 46, reduced visual acuity and reduced contrast sensitivity (measured as increased contrast sensitivity thresholds) 12, 13, 20, 25, 47, and RGC loss 16, 18–20, 24, 48 have been documented in various substrains of DBA/2 mice, while photoreceptor loss was minimal to none 18, 48, with normal levels of rhodopsin immunoreactivity and minimal photoreceptor loss restricted to cones possibly in areas where RGC loss is prominent18, however, thinning of the outer retinal layers as well as the overall retina was reported for 7-month and older DBA/2NNia mice.22 There are few reports that the optomotor response, which is of course a requirement for the determination of visuospatial thresholds, could not be observed in DBA/2 mice, independent of retina function and progression of glaucoma.19, 23 This discrepency may be dependent on the testing parameters used for this mouse model, such as acclimatization time, initial grating speed, testing time, etc., and was reviewed by us recently1.
Additionally, we observed that unspecific labelling of blood vessels during immunostaining experiments highlighted an increased vascularization in the INL, IPL and GCL of older, glaucomatous DBA/2 mice, compared to the younger, non-glaucomatous DBA/2 mice (Figure 1D). This retinal neovascularization has been documented before in DBA/2J mice that are 6 months and older and could be due to hypoxia resulting in the upregulation of vascular endothelial growth factor (VEGF), which has been interpreted as a physiological “revascularization” in the DBA/2J retina rather than a consequence of the glaucoma disease process.48
11-month old DBA/2 mice exhibit deficits in fERG function
ISCEV regulated fERG parameters30 indicated age-related differences between the older, glaucomatous and the younger, non-glaucomatous DBA/2 mice, such as the a-wave representing photoreceptor function (Fig. 1A, C) in the mixed rod-cone response to light stimuli, and development of a detectable a-wave in 11-month old DBA/2 mice only at higher light intensities when compared to young mice. Similalrly, the b-wave containing responses of interneurons such as bipolar cells (Fig 1B, D) indicated slower and smaller responses from rod bipolar cells, while responses of cone bipolar cells were unchanged with respect to response onset, but also had reduced amplitudes in older, glaucomatous DBA/2 mice. In addition, a higher flash intensity was needed for the older cohort to achieve a 50% maximal response in scotopic a-wave and b-wave when comparing their intensity/response function with those of younger mice. For the photopic response, a-wave and b-wave amplitudes were decreased, while intensity curve parameters were not significantly different. Previous studies have shown that the a-wave and b-wave parameters from fERGs either remained stable or exhibited a slight, statitistically not significant decrease with age in wild-type C57BL/6 mice during aging16, 17, 21, 22, but that a-wave and/or b-wave amplitudes21, 22 significantly declined with age in DBA/2 mice, confirming the present results.
Deficits measured in the a-wave and b-wave fERG response represent changes in the outer retina, INL and in their respective synaptic connections, 22 indicating that DBA/2 mice may have such retinal deficits with increasing glacuoma pathology. The b/a ratio representing a measure of signal transmission between photoreceptor and bipolar cells in response to light stimuli,24, 49, 50 for mice is typically greater than 1.651 with C57BL/6 mice exhibiting b/a ratios of 1.9–2.452 at 1 log cd·s/m2 flash intensity. Kjellstrom and colleagues reported the mean b/a ratio of 1–16 month-old C57BL/6mice as 1.8853 while Staropoli and colleagues measured a b/a ratio of 2.4 in 16 month-old wild-type mice.51 Calculating from mean a- and b-wave amplitudes published in Hazarny et al. 2009, estimated b/a ratios for C57BL/6mice remained stable with age, varying from 2.89 – 3.59 in two to 10 month-old animals. However, the b/a ratios in DBA/2J mice steadily increased from 2.71 at 2 months of age to 6.43 by the time animals were 10 months old.21 We found in the present study that four-month old DBA/2 mice had b/a ratios of 1.84 and 1.82 at flash intensities of 0.5 and 1 log cd·s/m2, which is in agreement with published values. At the same flash intensities, the older, 10 month-old DBA/2 mice had significantly increased b/a ratios (2.18 and 2.08, respectively, Fig. 3E) compared to the younger four-month old DBA/2 mice. Increased b/a ratios are indicative of altered outer retina function. Since only the rod and the mixed rod and cone functions were affected, it appears that scotopic vision dependent on rod photoreceptor signals is impaired in older DBA/2 mice. Previous studies found no obvious changes in the integrity or neuronal morphology of aging DBA/2J and C57BL/6 mice, however an age-dependent thinning of the OPL and structural changes in rod photoreceptor ribbon synapses were observed.54 Fernandez-Sánchez et al., showed that DBA/2J mice had outer retinal damage at all ages, the mose severe at the oldest measured age (16 months).55 Lastly, flicker reponses, representing bipolar cell activity, indicated in the present study that a signifiantly delayed b-wave occurs in older DBA/2 mice. Hazarny et al., 2009 compared this phenotype to young C57BL/6mice and determined that this delay indicating retinal dysfunction occurs at a young age in the DBA2 mouse.21 Hazarny and colleagues also showed decreased amplitudes in flicker responses when compared to C57BL/6 mice 33, while others observed little change in outer retinal function for DBA/2 mice.56
Decreased RGCs function in 11-month old DBA/2 mice
While nSTR and pSTR reflect RGC and amacrine cell responses 5, Smith et al. 2014 determined that the nSTR is both the more reliable and sensitive measure of RGC function in mice, when compared to other fERG parameters, such as pSTR, PhNR and OP amplitudes.7 Both pSTR and nSTR (Fig. 5A) are sensitive measurements that are easily saturated.5 Our finding that the pSTR measured at −4 log cd·s/m2 is reduced by 46.6% in 11-month old DBA/2 mice when compared to four-month old controls is in agreement with published data: pSTR was reduced by 39.2% in 9 month-old DBA/2J mice, by 68.4% in 12 month-old, 16 and by 75% in 15m old DBA/2 mice, when compared to 3m old DBA/2 mice 17, while no significant decrease with age was found for pSTR amplitudes of C57BL/6 mice 16,17. Alarcon-Martinez et al. 2010 determined that nSTR and pSTRs are reduced up to 40% in pigmented and up to 55% in albino mice two weeks after a transection of the optic nerve 3 and observed similar effects in albino and pigmented rats 2. This is in good agreement with data presented here, where we measured a nSTR reduction of 46% at −4 log cd·s/m2. As PhNR correlates closely with pSTRs 4, we expected to see, but found no difference between the four and 11-month old DBA/2 mice. This result could represent a specific property of the DBA/2 model differing from other mouse strains and disease models. Similarly, for models utilizing RGC loss induced by optic nerve transection both reduction of the PhNR 57 and no effect 58 were seen.
Changes in latency and amplitude of oscillatory potentials in 11-month old DBA/2 mice
OPs, wavelets on the ascending portion of the b-wave 8–10, reflect synaptic communication among components of the inner retina 21, bipolar, ganglion and amacrine cells.8, 10 OPs were detected at flash intensities higher than 0.5 for OP1 and −2 log cd·s/m2 for OP2-OP4, and they were indistinguishable from noise at lower flash intensities. We measured four peaks on the ascending part of the b-wave (Fig. 6A,B) as described previously in other studies. 7, 9 In the present study, OP1 was not detectable in 11-month old DBA/2 mice when providing the same low-intesity stimulus that elicited OP1 in four-month old mice. At 0.5 log cd·s/m2, a small OP1 wavelet was detected in 1 mouse (both eyes, n=2, indicated Fig 6. D). At stimulus intensities above 0.5 log cd·s/m2, OP1 was observable in 11-month old DBA/2 mice, however with increased latency and larger amplitude than in young mice. OP2 and 3 amplitudes did not differ between four and 11-month old DBA/2 mice at all flash intensities, however OP2 and 3 latencies were increased at lower flash intensities for 11-month old when compared to young mice. The OP4 amplitude was particularly affected in 11-month old DBA/2 mice: Amplitudes for OP4 peaks were similar for all flash intensities, unlike in the four-month old mice, who exhibited the typical flash-intensity dependent increase in OP4 amplitude. While in the present study, the sum of OPs was not statistically different between the two ages investigated, Harazny and colleagues measured an age-related decrease in OPs in DBA/2 mice 21 and Heiduschka et al. 2010 report that DBA/2J mice have smaller OP amplitudes under both photopic and scotopic conditions when compared to C57BL/6 mice of the same age.24 Therefore, while overall our data and those from other studies agree with respect to changes in latency and amplitude of oscillatory potentials in glaucomatous DBA/2 mice, differences in individual parameters and their respective degree of change appear to be dependent on the level of disease progression, which is correlated with age in DBA/2 mice, sub-strain and refence point for statistical comparisons, e.g. comparison with younger DBA/2 vs. with C57BL/6 mice of the same age.
Behavioral measures of visual performance correlate with fERG parameters measuring RGC function
The present study indicates that STR parameters determined at −4 log cd·s/m2 flash intensity correlated in a statistically significant manner with behavioral measures of visual performance, IOP and RGC numbers identifying the animals as glaucomatous: With STRs indicating amacrine cell and RGC function, the strong correlations of the pSTR with changes in visual acuity and contrast sensitivity, of the nSTR with IOP and RGC number, and of STR amplitude with visual acuity indicate visual impairment at the level of the inner retina. The strong correlation of nSTRs with RGC counts, but not with behavioral measures of visual performance combined with the strong correlation of the pSTR with visual acuity and contrast sensitivity, but not with RGC numbers potentially indicates that the nSTR is a more direct measure of the RGC response, while the pSTR is a function of the RGC response combined with the activity of other cells. Similalry, a selection of parameters of OPs are differentially correlated with visual acuity and/or contrast sensitivity. OP1, OP3 and OP4 amplitudes correlate with visual acuity while only the OP4 amplitude correlates with contrast sensitivity. These results, therefore, suggest that the nSTR and OP4 amplitude represent reproducible electrophysiological measures that can serve as surrogate markers of RGC function, and that pSTR and OP4 amplitude can serve as indicators of visual function involving RGC and other components of the visual signaling pathway as they are altered by the glaucoma disease process.
Summary
Here, we show that parameters of visual function that are commonly affected by glaucomatous retinopathy and its progression can be reproducibly correlated with measurements of RGC function using the non-invasive flash electroretinography. Based on these analyses, we propose the nSTR as direct measure of the decline in RGC numbers during glaucoma while pSTRs can serve as a measure in this field that includes RGC responses as well as interactions with other cells. In addition, we suggest that the OP4 amplitude can be employed as a good indicator of visual function in a glaucoma model as it is well correlated with both visual acuity and contrast sensitivity, whereas other parameters of OPs had a less robust or no predictive value. Future studies might shed additional light on how fERG parameters and behavioral measures of visual performance are linked mechanistically.
Acknowledgments:
The research presented in the present publication was supported in part by NIH grants AG027956 from NIH/NIA, RR027093 from NIH/NCRR, EY022774 and EY027005 from NIH/NEI (PK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research, the Vision Research Foundation of Kansas City (PK) and a departmental challenge grant by Research to Prevent Blindness (PK) is gratefully acknowledged. The authors thank Margaret, Richard and Sara Koulen for generous support and encouragement.
References
- 1.Grillo SL, Koulen P. Psychophysical testing in rodent models of glaucomatous optic neuropathy. Exp Eye Res 2015;141:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alarcon-Martinez L, de la Villa P, Aviles-Trigueros M, Blanco R, Villegas-Perez MP, Vidal-Sanz M. Short and long term axotomy-induced ERG changes in albino and pigmented rats. Mol Vis 2009;15:2373–2383. [PMC free article] [PubMed] [Google Scholar]
- 3.Alarcon-Martinez L, Aviles-Trigueros M, Galindo-Romero C, et al. ERG changes in albino and pigmented mice after optic nerve transection. Vision Res 2010;50:2176–2187. [DOI] [PubMed] [Google Scholar]
- 4.Chrysostomou V, Crowston JG. The photopic negative response of the mouse electroretinogram: reduction by acute elevation of intraocular pressure. Invest Ophthalmol Vis Sci 2013;54:4691–4697. [DOI] [PubMed] [Google Scholar]
- 5.Saszik SM, Robson JG, Frishman LJ. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. The Journal of physiology 2002;543:899–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bui BV, Fortune B. Ganglion cell contributions to the rat full-field electroretinogram. The Journal of physiology 2004;555:153–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith BJ, Wang X, Chauhan BC, Cote PD, Tremblay F. Contribution of retinal ganglion cells to the mouse electroretinogram. Doc Ophthalmol 2014;128:155–168. [DOI] [PubMed] [Google Scholar]
- 8.Lei B, Yao G, Zhang K, Hofeldt KJ, Chang B. Study of rod- and cone-driven oscillatory potentials in mice. Invest Ophthalmol Vis Sci 2006;47:2732–2738. [DOI] [PubMed] [Google Scholar]
- 9.Hancock HA, Kraft TW. Oscillatory potential analysis and ERGs of normal and diabetic rats. Invest Ophthalmol Vis Sci 2004;45:1002–1008. [DOI] [PubMed] [Google Scholar]
- 10.Dong CJ, Agey P, Hare WA. Origins of the electroretinogram oscillatory potentials in the rabbit retina. Vis Neurosci 2004;21:533–543. [DOI] [PubMed] [Google Scholar]
- 11.McKinnon SJ, Schlamp CL, Nickells RW. Mouse models of retinal ganglion cell death and glaucoma. Experimental Eye Research 2009;88:816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montgomery CL, Keereetaweep J, Johnson HM, Grillo SL, Chapman KD, Koulen P. Changes in Retinal N-Acylethanolamines and their Oxylipin Derivatives During the Development of Visual Impairment in a Mouse Model for Glaucoma. Lipids 2016;51:857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaja S, Naumchuk Y, Grillo SL, Borden PK, Koulen P. Differential up-regulation of Vesl-1/Homer 1 protein isoforms associated with decline in visual performance in a preclinical glaucoma model. Vision Res 2014;94:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grillo SL, Keereetaweep J, Grillo MA, Chapman KD, Koulen P. N-Palmitoylethanolamine depot injection increased its tissue levels and those of other acylethanolamide lipids. Drug design, development and therapy 2013;7:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burroughs SL, Kaja S, Koulen P. Quantification of deficits in spatial visual function of mouse models for glaucoma Invest Ophthalmol Vis Sci 2011;Article In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez de Lara MJ, Santano C, Guzman-Aranguez A, et al. Assessment of inner retina dysfunction and progressive ganglion cell loss in a mouse model of glaucoma. Exp Eye Res 2014;122:40–49. [DOI] [PubMed] [Google Scholar]
- 17.Perez de Lara MJ, Guzman-Aranguez A, de la Villa P, Diaz-Hernandez JI, Miras-Portugal MT, Pintor J. Increased levels of extracellular ATP in glaucomatous retinas: Possible role of the vesicular nucleotide transporter during the development of the pathology. Mol Vis 2015;21:1060–1070. [PMC free article] [PubMed] [Google Scholar]
- 18.Atorf J, Scholz M, Garreis F, Lehmann J, Brauer L, Kremers J. Functional protective effects of long-term memantine treatment in the DBA/2J mouse. Doc Ophthalmol 2013;126:221–232. [DOI] [PubMed] [Google Scholar]
- 19.Barabas P, Huang W, Chen H, et al. Missing optomotor head-turning reflex in the DBA/2J mouse. Invest Ophthalmol Vis Sci 2011;52:6766–6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rangarajan KV, Lawhn-Heath C, Feng L, Kim TS, Cang J, Liu X. Detection of visual deficits in aging DBA/2J mice by two behavioral assays. Current eye research 2011;36:481–491. [DOI] [PubMed] [Google Scholar]
- 21.Harazny J, Scholz M, Buder T, Lausen B, Kremers J. Electrophysiological deficits in the retina of the DBA/2J mouse. Doc Ophthalmol 2009;119:181–197. [DOI] [PubMed] [Google Scholar]
- 22.Bayer AU, Neuhardt T, May AC, et al. Retinal morphology and ERG response in the DBA/2NNia mouse model of angle-closure glaucoma. Invest Ophthalmol Vis Sci 2001;42:1258–1265. [PubMed] [Google Scholar]
- 23.Puk O, Dalke C, Hrabe de Angelis M, Graw J. Variation of the response to the optokinetic drum among various strains of mice. Front Biosci 2008;13:6269–6275. [DOI] [PubMed] [Google Scholar]
- 24.Heiduschka P, Julien S, Schuettauf F, Schnichels S. Loss of retinal function in aged DBA/2J mice - New insights into retinal neurodegeneration. Exp Eye Res 2010;91:779–783. [DOI] [PubMed] [Google Scholar]
- 25.Burroughs SL, Kaja S, Koulen P. Quantification of deficits in spatial visual function of mouse models for glaucoma. Investigative ophthalmology & visual science 2011;52:3654–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci 2005;22:677–684. [DOI] [PubMed] [Google Scholar]
- 27.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci 2004;45:4611–4616. [DOI] [PubMed] [Google Scholar]
- 28.Prusky GT, Alam NM, Douglas RM. Enhancement of vision by monocular deprivation in adult mice. J Neurosci 2006;26:11554–11561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Q, Stell WK. Die Fledermaus: regarding optokinetic contrast sensitivity and light-adaptation, chicks are mice with wings. PloS one 2013;8:e75375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marmor MF, Fulton AB, Holder GE, et al. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol 2009;118:69–77. [DOI] [PubMed] [Google Scholar]
- 31.Heckenlively JR, Arden GB. Principles and practice of clinical electrophysiology of vision. St. Louis: Mosby Year Book; 1991:xv, 829 p. [Google Scholar]
- 32.Choh V, Gurdita A, Tan B, Prasad RC, Bizheva K, Joos KM. Short-Term Moderately Elevated Intraocular Pressure Is Associated With Elevated Scotopic Electroretinogram Responses. Invest Ophthalmol Vis Sci 2016;57:2140–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abd-El-Barr MM, Pennesi ME, Saszik SM, et al. Genetic dissection of rod and cone pathways in the dark-adapted mouse retina. J Neurophysiol 2009;102:1945–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeiffer-Guglielmi B, Fleckenstein B, Jung G, Hamprecht B. Immunocytochemical localization of glycogen phosphorylase isozymes in rat nervous tissues by using isozyme-specific antibodies. J Neurochem 2003;85:73–81. [DOI] [PubMed] [Google Scholar]
- 35.Haverkamp S, Wassle H, Duebel J, et al. The primordial, blue-cone color system of the mouse retina. J Neurosci 2005;25:5438–5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wassle H, Regus-Leidig H, Haverkamp S. Expression of the vesicular glutamate transporter vGluT2 in a subset of cones of the mouse retina. The Journal of comparative neurology 2006;496:544–555. [DOI] [PubMed] [Google Scholar]
- 37.Zhang DR, Yeh HH. Protein kinase C-like immunoreactivity in rod bipolar cells of the rat retina: a developmental study. Vis Neurosci 1991;6:429–437. [DOI] [PubMed] [Google Scholar]
- 38.Nadal-Nicolas FM, Jimenez-Lopez M, Sobrado-Calvo P, et al. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest Ophthalmol Vis Sci 2009;50:3860–3868. [DOI] [PubMed] [Google Scholar]
- 39.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baggaley AR. Intermediate Correlational Methods: pp. 211 John Wiley & Sons: New York; 1964:8º. [Google Scholar]
- 41.Bartlett RF. Linear Modelling of Pearson’s Product Moment Correlation Coefficient: An Application of Fisher’s $z$-Transformation. Journal of the Royal Statistical Society Series D (The Statistician) 1993;42:45–53. [Google Scholar]
- 42.Woodward WR, Choi D, Grose J, et al. Isoflurane is an effective alternative to ketamine/xylazine/acepromazine as an anesthetic agent for the mouse electroretinogram. Doc Ophthalmol 2007;115:187–201. [DOI] [PubMed] [Google Scholar]
- 43.Charng J, Nguyen CT, He Z, et al. Conscious Wireless Electroretinogram and Visual Evoked Potentials in Rats. PLoS ONE 2013;8:e74172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nair G, Kim M, Nagaoka T, et al. Effects of common anesthetics on eye movement and electroretinogram. Doc Ophthalmol 2011;122:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baltan S, Inman DM, Danilov CA, Morrison RS, Calkins DJ, Horner PJ. Metabolic vulnerability disposes retinal ganglion cell axons to dysfunction in a model of glaucomatous degeneration. J Neurosci 2010;30:5644–5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inman DM, Sappington RM, Horner PJ, Calkins DJ. Quantitative correlation of optic nerve pathology with ocular pressure and corneal thickness in the DBA/2 mouse model of glaucoma. Invest Ophthalmol Vis Sci 2006;47:986–996. [DOI] [PubMed] [Google Scholar]
- 47.Zhou X, Li F, Kong L, Chodosh J, Cao W. Anti-inflammatory effect of pigment epithelium-derived factor in DBA/2J mice. Mol Vis 2009;15:438–450. [PMC free article] [PubMed] [Google Scholar]
- 48.Schuettauf F, Rejdak R, Walski M, et al. Retinal neurodegeneration in the DBA/2J mouse-a model for ocular hypertension. Acta Neuropathol 2004;107:352–358. [DOI] [PubMed] [Google Scholar]
- 49.Sergeev YV, Caruso RC, Meltzer MR, Smaoui N, MacDonald IM, Sieving PA. Molecular modeling of retinoschisin with functional analysis of pathogenic mutations from human X-linked retinoschisis. Hum Mol Genet 2010;19:1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park TK, Wu Z, Kjellstrom S, et al. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther 2009;16:916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staropoli JF, Haliw L, Biswas S, et al. Large-scale phenotyping of an accurate genetic mouse model of JNCL identifies novel early pathology outside the central nervous system. PLoS One 2012;7:e38310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaucher D, Chiappore JA, Paques M, et al. Microglial changes occur without neural cell death in diabetic retinopathy. Vision Res 2007;47:612–623. [DOI] [PubMed] [Google Scholar]
- 53.Kjellstrom S, Bush RA, Zeng Y, Takada Y, Sieving PA. Retinoschisin gene therapy and natural history in the Rs1h-KO mouse: long-term rescue from retinal degeneration. Invest Ophthalmol Vis Sci 2007;48:3837–3845. [DOI] [PubMed] [Google Scholar]
- 54.Fuchs M, Scholz M, Sendelbeck A, et al. Rod photoreceptor ribbon synapses in DBA/2J mice show progressive age-related structural changes. PLoS One 2012;7:e44645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez-Sanchez L, de Sevilla Muller LP, Brecha NC, Cuenca N. Loss of outer retinal neurons and circuitry alterations in the DBA/2J mouse. Invest Ophthalmol Vis Sci 2014;55:6059–6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagaraju M, Saleh M, Porciatti V. IOP-dependent retinal ganglion cell dysfunction in glaucomatous DBA/2J mice. Invest Ophthalmol Vis Sci 2007;48:4573–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li B, Barnes GE, Holt WF. The decline of the photopic negative response (PhNR) in the rat after optic nerve transection. Doc Ophthalmol 2005;111:23–31. [DOI] [PubMed] [Google Scholar]
- 58.Mojumder DK, Sherry DM, Frishman LJ. Contribution of voltage-gated sodium channels to the b-wave of the mammalian flash electroretinogram. The Journal of physiology 2008;586:2551–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]