Abstract
Background
Extensive studies have been performed on the role of monoaminergic neuronal systems in rodents exposed to social defeat stress as adults. In the present study, we investigated the role of monoaminergic neuronal systems in the impairment of social behaviors induced by social defeat stress exposure as juveniles.
Methods
Juvenile, male C57BL/6J mice were exposed to social defeat stress for 10 consecutive days. From 1 day after the last stress exposure, desipramine, sertraline, and aripiprazole were administered for 15 days. Social behaviors were assessed at 1 and 15 days after the last stress exposure. Monoamine turnover was determined in specific regions of the brain in the mice exposed to the stress.
Results
Stress exposure as juveniles induced the impairment of social behaviors in adolescent mice. In mice that showed impairment of social behaviors, turnover of serotonin and dopamine, but not noradrenaline, was decreased in specific brain regions. Acute and repeated administration of desipramine, sertraline, and aripiprazole failed to attenuate the impairment of social behaviors, whereas repeated administration of a combination of sertraline and aripiprazole showed additive attenuating effects.
Conclusions
These findings suggest that social defeat stress exposure as juveniles induces the treatment-resistant impairment of social behaviors in adolescents through dysfunction in the serotonergic and dopaminergic neuronal systems. The combination of sertraline and aripiprazole may be used as a new treatment strategy for treatment-resistant stress-related psychiatric disorders in adolescents with adverse juvenile experiences.
Keywords: social defeat stress, juvenile, adolescent, social behaviors, monoaminergic neuronal system
Significance Statement
Monoaminergic neuronal systems have attracted the attention of researchers with regard to the impairment of social behaviors induced by social defeat stress exposure as adults. However, the importance of monoaminergic neuronal systems in the impairment of social behaviors induced by exposure to social defeat stress as juveniles remains poorly understood; thus, in the present study, we investigated the role of monoaminergic neuronal systems in the impairment of social behaviors induced by social defeat stress exposure as juveniles. Exposure to social defeat stress as juveniles leads to the treatment-resistant impairment of social behaviors in adolescents, because of dysfunction of the serotonergic and dopaminergic neuronal systems. Repeated administration of a combination of sertraline and aripiprazole showed additive effects in attenuating this impairment of social behaviors. This may be useful as a new treatment strategy for treatment-resistant stress-related psychiatric disorders in adolescents with adverse juvenile experiences.
Introduction
Adverse juvenile experiences, including physical or sexual violence and neglect, often induce adverse mental health outcomes later in life (Afifi, 2011; Annerback et al., 2012; McKenzie and Scott, 2012). Epidemiological studies have demonstrated that adverse juvenile experiences increase the risk for stress-related psychiatric disorders, particularly major depressive disorder (MDD), anxiety disorder, and posttraumatic stress disorder (Weber et al., 2008; Weich et al., 2009; McLaughlin et al., 2010). These psychiatric disorders induced by adverse juvenile experiences frequently involve marked dysfunction in social activity during adolescence and adulthood (Sandi and Haller, 2015).
The monoaminergic neuronal system has attracted increasing attention of researchers in the field of stress-related psychiatric disorders (Flugge, 2000; Pittenger and Duman, 2008; Krystal and Neumeister, 2009). Polymorphism of the serotonin (5-HT) transporter (5-HTTLPR genotype) has been reported to be a susceptibility factor for posttraumatic stress disorder in the interaction between adult traumatic events and childhood adversity (Steckler and Risbrough, 2012). Further, monoaminergic neuronal dysregulation may contribute vulnerability to stress as a factor in the development of anxiety disorders, because 5-HT reuptake inhibitors and dual 5-HT/noradrenaline (NA) reuptake inhibitors are effective in treating anxiety disorders (Morilak and Frazer, 2004). Low CSF levels of 5-hydroxyindoleacetic acid (5-HIAA), 3-methoxy-4-hydroxyphenylethylene glycol (MHPG), and homovanilic acid (HVA) have been hypothesized to be involved in MDD, although the published literature is contradictory (Placidi et al., 2001). Extensive investigation on the role of monoaminergic neuronal systems has also been performed in rodents exposed to social defeat stress as adults (Krishnan et al., 2007; Cao et al., 2010; Chen et al., 2012; Boyarskikh et al., 2013).
Mice exposed to social defeat stress as adults display the impairment of social behaviors (Berton et al., 2006; Tsankova et al., 2006). Previous studies have shown that tryptophan hydroxylase 2 knockin mice, which show 60% to 80% reduction in brain 5-HT, have increased susceptibility to social defeat stress (Nakayama et al., 2003). The impairment of social behaviors induced by social defeat stress exposure is dependent on the mesolimbic dopamine (DA) circuit (Tanaka et al., 2012). In addition, NA transporter-knockout mice resist the impairment of social behaviors induced by social defeat stress exposure (Haenisch et al., 2009). These findings suggest that brain monoaminergic neuronal systems are involved in the impairment of social behaviors induced by social defeat stress exposure. We previously found that juvenile mice were more vulnerable to the impairment of social behaviors induced by social defeat stress exposure than adult mice (Mouri et al., 2018). However, the role of monoaminergic neuronal systems in the impairment of social behaviors induced by social defeat stress exposure as juveniles remains unclear.
The present study was designed to investigate the role of monoaminergic neuronal systems in the impairment of social behaviors induced by social defeat stress exposure as juveniles. We determined the functional and neurochemical changes in the monoaminergic neuronal systems of mice exposed to social defeat stress as juveniles using biochemical techniques. We also investigated the effect of antidepressants and aripiprazole on the impairment of social behaviors in adolescent mice exposed to social defeat stress as juveniles.
Materials and Methods
Animals
Male C57BL/6J and ICR mice were obtained from Japan SLC, Inc. Juvenile, male C57BL/6J mice (3 weeks old) were used to exposure to social defeat stress. Aggressive, male ICR mice (over 7 weeks old) for social defeat stress were screened based on the duration of attacks on C57BL/6J mice, with more than 1/10 minutes as the inclusion criterion. Unfamiliar target male ICR mice (over 4–6 weeks old) were used for the social interaction test. They were housed in plastic cages in a regulated environment (23°C ±1°C, 50±5% humidity), with a 12-hour-light/-dark cycle (lights on at 9:00 am). Food (CE2; Clea Japan Inc.) and tap water were available ad libitum. All experiments were conducted in accordance with the Guidelines for Animal Experiments of the Nagoya University Graduate School of Medicine. Procedures involving animals and their care were conformed to the international guidelines set out in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Drug Administration
Desipramine hydrochloride, sertraline hydrochloride, and aripiprazole were purchased from Sigma-Aldrich, Tokyo Chemical Industry Co, Ltd, and Wako Pure Chemical Industries, Ltd, respectively. Desipramine and sertraline were dissolved in distilled water. Aripiprazole was dissolved in 100% acetic acid and diluted with distilled water.
From 1 day after the last social defeat stress exposure, i.p. administration of 10 and 20 mg/kg desipramine, 5 and 10 mg/kg sertraline, or 0.003 and 0.01 mg/kg aripiprazole was commenced; this treatment was performed once a day for 15 days. The administration volume was 10 mL/kg per mouse.
Social Defeat Stress
Social defeat stress exposure was carried out according to the method outlined in previous reports (Berton et al., 2006; Krishnan et al., 2007) with minor modifications. Prior to social defeat stress, an aggressive ICR mouse was habituated to social defeat stress cages for 10 minutes. C57BL/6J mice (3 weeks old) were exposed to a different aggressive ICR mouse for 10 minutes each day for 10 consecutive days. The pairing of defeated and aggressive mice was randomized daily to minimize the effects of variability in aggression that the mice were exposed to.
Defeat was defined as the display of defensive behaviors by C57BL/6J mice, such as escape and submissive postures during physical attacks by an aggressive mouse. Submissive posture was defined as standing upright with the belly exposed to the aggressor. The duration of defensive behaviors was recorded according to our previous report (Mouri et al., 2018). After 10 minutes, an aggressive mouse was separated from the defeated mouse to avoid habituation to the defeated mouse and placed in its group-housed home cage for the next 24 hours.
We previously found that mice exposed to a nonaggressive ICR mouse (undefeated mice) as well as an empty cage (control mice) for 10 consecutive days showed significantly increased duration at the interaction zone in the second session compared with that in the first session (Mouri et al., 2018). Thus, it is unlikely that defeated mice were habituated to expose ICR mouse during social defeat stress and did not approach familiar target ICR mouse.
Control mice were exposed to an empty cage as described in our previous report (Mouri et al., 2018).
Social Interaction Test
The adolescent mice (4 or 6 weeks old) were subjected to the social interaction test at 1 and 15 days after the last stress exposure. The social interaction test was performed from 10:00 am to 5:00 pm in a sound-attenuated room, as described in our previous report (Mouri et al., 2018). The apparatus consisted of an open gray nonreflecting acrylic box (W42×D42×H30 cm) and the transparent Plexiglas enclosure (W10×D6.5×H30 cm) with 30 holes (10 mm in diameter). A lightbulb (54 W), not directly seen by the mouse, was attached to the upper part of the apparatus and provided constant illumination of approximately 20 lux.
The social interaction test consisted of 2 sessions: in the first session, the mouse was allowed to freely explore and habituate to the test environment for 30 minutes in the absence of an unfamiliar target ICR mouse, in consistent with a previous report (Mouri et al., 2018). This was done to reduce the time spent in the interaction zone by exploring the apparatus itself during the second session. The second session commenced 1 minute after the first session, and the mouse was returned to the apparatus for 5 minutes in the presence of an unfamiliar target ICR mouse. During the test, the time spent in the interaction zone (6 cm width) was recorded for the last 5 minutes of the first (target absent) and for the 5 minutes of the second (target present) sessions, using a video-tracking system (EthoVision XT; Noldus Information Technology).
Preparation of Brain Samples
Each mouse was killed immediately before or soon after the social interaction test. Their brains were rapidly removed and the prefrontal cortex (PFC), nucleus accumbens (NAc), hippocampus (HIP), and amygdala (AMG) were dissected out on an ice-cold plate according to the atlas (Paxinos and Franklin, 2004). Each tissue sample was quickly frozen on dry ice, weighed, and stored in a deep freezer at -80°C until assay.
Determination of Monoamines and Their Metabolites
The concentrations of monoamines and their metabolites were determined using a high-performance liquid chromatography system with an electrochemical detector (Eicom) as described in previous reports (Noda et al., 1997, 1998). Briefly, each frozen tissue sample was homogenized with an ultrasonic processor (475 W, Model XL2020, Heat Systems Inc.) in 350 μL of 0.2 M perchloric acid containing isoproterenol (internal standard). The homogenate was placed in ice for 30 minutes and then centrifuged at 20000×g for 15 minutes at 0°C. The supernatant was mixed with 1 M sodium acetate to adjust the pH to 3.0 and then injected into a liquid chromatography system equipped with a reversed-phase ODS-column (3×150 mm, diameter of stationary phase grains 5 μm: Eicompak SC-5ODS, Eicom) and an electrochemical detector (model ECD-700, Eicom). The column temperature was maintained at 25°C, and the detector potential was set at +750 mV. The mobile phase consists of 0.1 M citric acid and 0.1 M sodium acetate, pH 3.5, containing 15% methanol, 220 mg/L sodium-l-octanesulfonate, and 5 mg/L ethylenediaminetetraacetic acid; the flow rate was 0.5 mL/min. Data were collected and analyzed with the PowerChrom v2.6.4 software (eDAQ).
Statistical Analysis
All results are expressed as the mean±SEM for each group. Statistical significance was determined using 1-way or 2-way ANOVA followed by Bonferroni’s test. The Student’s t test was used to compare 2 sets of data. P <.05 was taken to indicate significance. Data were analyzed with SPSS 24 software (IBM).
Results
The Impairment of Social Behaviors in Adolescent Mice Exposed to Social Defeat Stress as Juveniles
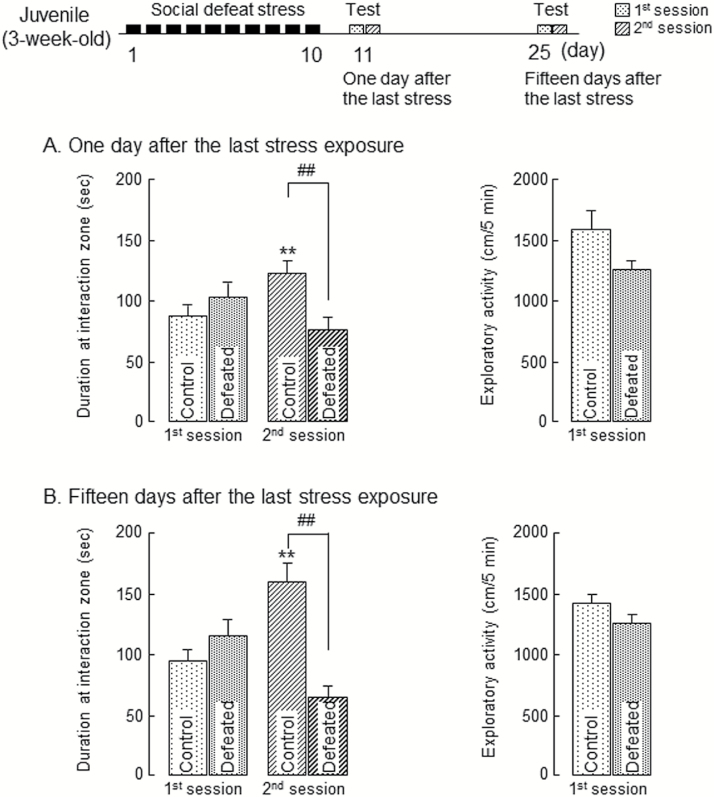
Adolescent, male C57BL/6J mice were exposed to social defeat stress as juveniles for 10 consecutive days. When juvenile mice body weight was measured immediately after the stress exposure of 10 consecutive days, the stress exposure did not affect body weight gain (data not shown), in consistent with a previous report (Mouri et al., 2018). One day after the last stress exposure, mice were subjected to the social interaction test. In the first session without an unfamiliar ICR mouse, there was no significant difference between groups in the time spent in the interaction zone and engaging in exploratory activity within the apparatus (Figure 1A). In the second session, control mice approached the unfamiliar target ICR mouse and showed significantly increased time spent in the interaction zone than in the first session (Figure 1A). However, in the second session, the mice exposed to the stress as juveniles for 10 consecutive days spent significantly less time in the interaction zone than the control group, although there were no difference in exploratory activity between control and defeated groups (Figure 1A).
Figure 1.
The impairment of social behaviors in adolescent mice exposed to social defeat stress as juveniles. (A) Duration at interaction zone (left) and exploratory activity (right) 1 day after the last stress exposure. (B) Duration at interaction zone (left) and exploratory activity (right) 15 days after the last stress exposure. Each column represents the mean±SEM (n=12–15). Two-way ANOVA: duration at interaction zone: (A) Fdefeat (1, 25)=2.10, P=.16; Fsession (1, 25)=1.08, P=.31; Fdefeat × session (1, 25)=13.7, P<.01. (B) Fdefeat (1, 25)=7.16, P=.13; Fsession (1, 25)=0.57, P=.46; Fdefeat × session (1, 25)=22.3, P<.01. **P<.01 vs corresponding control in the first session (Student’s t test). ##P<.01 vs control group (Student’s t test). Control: mice exposed to an empty cage, defeated: mice exposed to an aggressive ICR mouse.
When tested 15 days after the last stress exposure, mice exposed to the stress as juveniles still spent less time in the interaction zone in the second session, although there was no difference in exploratory activity between control and defeated groups (Figure 1B). There was also no significant difference between groups in the time spent in the interaction zone and engaging in exploratory activity within the apparatus in the first session (Figure 1B). These results indicate that social defeat stress exposure as juveniles induces the persistent impairment of social behaviors in adolescents.
Changes in Monoamine Metabolisms in Brain Regions of Adolescent Mice Exposed to Social Defeat Stress as Juveniles
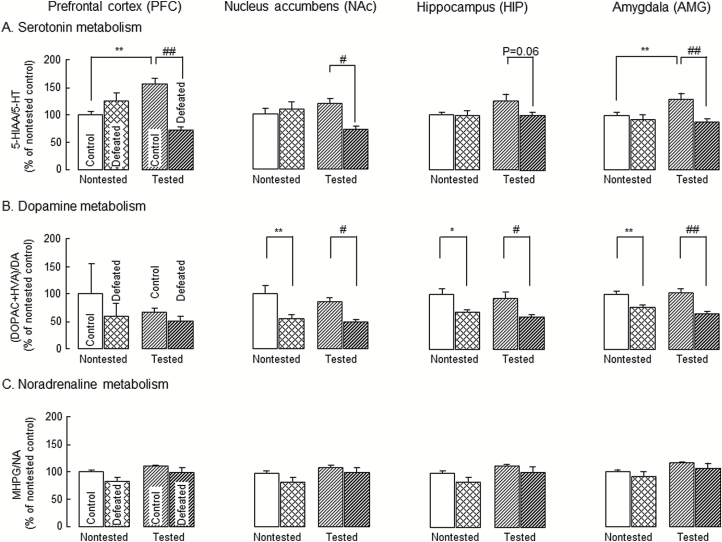
To examine the influence of social defeat stress exposure as juveniles on monoaminergic neuronal systems, we measured the concentrations of monoamines and their metabolites in the PFC, NAc, HIP, and AMG immediately before (nontested group) or after (tested group) the social interaction test (Table 1). The ratios of 5-HIAA/5-HT, [3, 4-dihydroxyphenyl acetic acid (DOPAC) + HVA]/DA, and MHPG/NA, which are used as indices of the 5-HT, DA, and NA turnover rates, were calculated in the PFC, NAc, HIP, and AMG of the nontested and tested groups (Figure 2).
Table 1.
Changes in Concentration of Monoamines and Their Metabolites in the Discrete Brain Regions of Adolescent Mice Exposed to Social Defeat Stress and Subjected to Social Interaction Test 1 Day after the Last Social Defeat Stress Exposure
| Region | Group | 5-HT | 5-HIAA | Dopamine | DOPAC | HVA | Noradorenaline | MHPG |
|---|---|---|---|---|---|---|---|---|
| Prefrontal cortex (PFC) | Nontested | |||||||
| Control | 202.1±7.2 | 208.6±5.9 | 96.9±27.9 | 87.4±14.8 | 73.5±6.6 | 246.6±2.6 | 50.0±1.6 | |
| Defeated | 193.1±19.1 | 229.8±9.2 | 114.7±27.1 | 76.4±11.0 | 85.0±6.8 | 275.6±8.2* | 53.5±5.7 | |
| Tested | ||||||||
| Control | 190.9±11.2 | 305.6±16.1** | 122.0±48.6 | 117.1±25.7 | 113.1±11.4 | 266.8±7.1 | 65.8±2.4 | |
| Defeated | 256.9±7.0## | 207.1±15.9## | 153.9±49.3 | 98.7±35.9 | 106.4±15.8 | 292.1±8.2 | 72.0±8.1 | |
| Nucleus accumbens (NAc) | Nontested | |||||||
| Control | 599.3±51.4 | 348.2±11.3 | 5839.0±445.8 | 1279.5±97.9 | 531.2±40.8 | 174.6±25.9 | 71.0±2.4 | |
| Defeated | 549.7±55.2 | 336.5±18.4 | 6711.7±664.9 | 905.4±77.07 | 476.7±62.2 | 245.2±38.4 | 81.7±6.2 | |
| Tested | ||||||||
| Control | 794.2±147.2 | 550.7±70.0** | 6845.1±708.8 | 1456.0±162.9 | 795.2±91.6* | 305.0±54.2 | 108.1±14.7* | |
| Defeated | 846.9±48.5 | 373.5±21.2# | 7368.4±355.2 | 764.2±54.2## | 647.7±28.8 | 342.5±47.1 | 114.4±8.5 | |
| Hippocampus (HIP) | Nontested | |||||||
| Control | 286.4±4.5 | 400.0±12.2 | 11.2±1.6 | 11.6±0.8 | 30.1±1.4 | 278.0±6.5 | 69.1±1.6 | |
| Defeated | 305.7±10.4 | 413.4±20.0 | 15.5±0.8 | 13.6±1.6 | 28.3±1.1 | 334.7±6.4* | 69.7±6.3 | |
| Tested | ||||||||
| Control | 271.9±26.2 | 455.7±36.0 | 14.5±1.6 | 15.1±2.6 | 37.4±3.5 | 263.1±17.0 | 73.0±5.1 | |
| Defeated | 303.4±15.4 | 411.1±19.4 | 21.3±2.2# | 14.6±1.8 | 34.3±1.5 | 328.8±16.2## | 80.8±1.6 | |
| Amygdala (AMG) | Nontested | |||||||
| Control | 426.9±8.1 | 494.5±16.4 | 265.6±22.9 | 154.8±17.9 | 82.4±7.8 | 254.7±4.4 | 72.3±1.7 | |
| Defeated | 423.2±14.6 | 451.0±20.8 | 311.8±42.9 | 132.7±16.5 | 78.1±9.4 | 293.4±8.2 | 76.6±5.3 | |
| Tested | ||||||||
| Control | 427.5±12.7 | 636.7±29.4** | 279.1±18.5 | 153.9±12.3 | 96.9±5.3 | 270.1±6.4 | 88.5±2.2 | |
| Defeated | 447.4±27.1 | 460.3±31.4## | 457.6±76.8 | 155.3±27.8 | 107.4±14.4 | 306.4±17.8 | 92.9±7.6 | |
Values are expressed as ng/g wet weight and are the means±SEM (n=8–9). The amounts of 5-HT, dopamine, noradrenaline, and their metabolites (5-HIAA, DOPAC, HVA, and MHPG) in the discrete brain regions were determined. Two-way ANOVA; 5-HT: Fbrain region (3, 120)=95.8, P<.01; Fgroup (3, 120)=4.18, P<.01; Fbrain region × group (9, 120)=2.63, P<.01, 5-HIAA: Fbrain region (3, 120)=77.0, P<.01; Fgroup (3, 120)=22.8, P<.01; Fbrain region × group (9, 120)=2.09, P<.05, dopamine: Fbrain region (3, 120)=535.7, P<.01; Fgroup (3, 120)=1.68, P=.18; Fbrain region × group (9, 120)=1.14, P=.34, DOPAC: Fbrain region (3, 120)=363.8, P<.01; Fgroup (3, 120)=9.76, P<.01; Fbrain region × group (9, 120)=8.78, P<.01, HVA: Fbrain region (3, 120)=312.4, P<.01; Fgroup (3, 120)=7.70, P<.01; Fbrain region × group (9, 120)=4.53, P<.01, noradrenaline: Fbrain region (3, 120)=0.19, P=.90; Fgroup (3, 120)=8.47, P<.01; Fbrain region × group (9, 120)=1.17, P=.32, MHPG: Fbrain region (3, 120)=21.0, P<.01; Fgroup (3, 120)=13.6, P<.01; Fbrain region × group (9, 120)=1.21, P=.29. *P<.05, **P<.01 vs non-tested control group, #P<.05, ##P<.01 vs tested control group (Bonferroni’s test). Nontested, mice sacrificed without performing the social interaction test; tested, mice sacrificed immediately after social interaction test; control, mice exposed to an empty cage; defeated, mice exposed to an aggressive ICR mouse.
Figure 2.
Changes in monoamine metabolisms in discrete brain regions of adolescent mice exposed to social defeat stress as juveniles. Changes in serotonin (A), dopamine (B), and noradrenaline (C) utilizations in brain regions of adolescent mice exposed to social defeat stress as juveniles. Data are expressed as a percentage of nontested control group (set at 100%). Each column represents the mean±SEM (n=8–9). Other details are shown in Table 1. Two-way ANOVA: (A) Fbrain region (3, 120)=2.21, P=.09; Fgroup (3, 120)=19.9, P<.01; Fbrain region × group (9, 120)=1.90, P=.60. (B) Fbrain region (3, 120)=1.02, P=.39; Fgroup (3, 120)=7.00, P<.01; Fbrain region × group (9, 120)=0.28, P=.98. (C) Fbrain region (3, 120)=6.06, P<.01; Fgroup (3, 120)=2.94, P=.36; Fbrain region × group (9, 120)=1.20 P=.30. *P<.05, **P<.01 vs nontested control group, #P<.05, ##P<.01 vs tested control group (Bonferroni’s test). Nontested: mice sacrificed without the social interaction test, tested: mice sacrificed immediately after social interaction test, control: mice exposed to an empty cage, defeated: mice exposed to an aggressive ICR mouse.
A significant increase in the 5-HIAA/5-HT ratio (5-HT metabolism) was observed in the PFC and AMG of the tested control group compared with that of the nontested control group (Figure 2A). Such significant increase in the 5-HIAA/5-HT ratio was not observed in the PFC and AMG of the tested defeated group (Figure 2A). In the PFC, NAc, and AMG, there was a significant decrease in the 5-HIAA/5-HT ratio in the tested defeated group compared with that in tested control group (Figure 2A). There were no significant changes in the 5-HIAA/5-HT ratio in the HIP when all groups were compared (Figure 2A). No changes in the (DOPAC + HVA)/DA ratio (DA metabolism) were observed in the tested control group (Figure 2B), whereas the ratio in the NAc, HIP, and AMG was significantly decreased in the both nontested and tested defeated groups compared with that in the corresponding control groups, respectively (Figure 2B). There were no significant changes in the MHPG/NA ratio (NA metabolism) in the all brain regions when all groups were compared (Figure 2C).
Significant changes in the concentrations of monoamines and their metabolites were observed as follows: the 5-HIAA concentration in the PFC, NAc, and AMG was significantly increased in the tested control group compared with that in the nontested control group. The increase in 5-HIAA concentration in the PFC, NAc, and AMG was significantly decreased in the tested defeated group compared with the tested control group. The 5-HT concentration in the PFC was significantly increased in the tested defeated group compared with that in the tested control group. The HVA concentration in the NAc was significantly increased in the tested control group compared with that in the nontested control group. The DA and DOPAC concentrations in the HIP and NAc were significantly increased and decreased, respectively, in the tested defeated group compared with those in the tested control group. The NA concentration in the PFC or HIP and the MHPG concentration in the NAc were significantly increased in the nontested defeated and the tested control groups, respectively, compared with those of the nontested control group. Lastly, the NA concentration in the HIP was significantly increased in the tested defeated group compared with that in the tested control group (Table 1).
Effects of Antidepressants and Aripiprazole on the Impairment of Social Behaviors in Mice Exposed to Social Defeat Stress as Juveniles
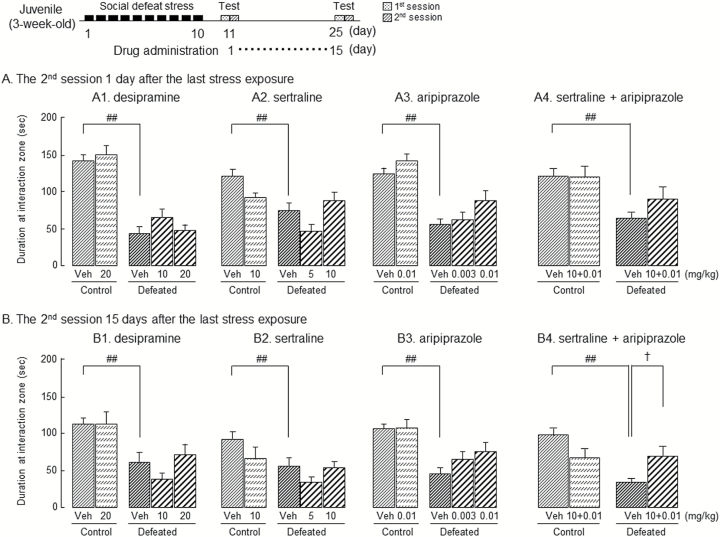
To investigate the role of monoaminergic neuronal systems in the impairment of social behaviors induced by social defeat stress exposure as juveniles, desipramine (10 and 20 mg/kg i.p.), a NA reuptake inhibitor; sertraline (5 and 10 mg/kg i.p.), a selective 5-HT reuptake inhibitor; and aripiprazole (0.003 and 0.01 mg/kg i.p.), a DA receptor partial agonist, were administered once a day from 1 day after social defeat stress exposure for 15 days (Figure 3A). The doses used followed previous publications; desipramine at 20 mg/kg (p.o.) and sertraline at 10 mg/kg (i.p.) attenuated depressive-like behaviors in a forced swim test (Noda et al., 1997; Mouri et al., 2012). Aripiprazole at 0.03 mg/kg (p.o.) attenuated phencyclidine-induced the impairment of recognition memory (Nagai et al., 2009); however, it did not affect locomotor activity at 0.01 mg/kg (i.p.) (Bourin et al., 2009).
Figure 3.
Effects of antidepressants and aripiprazole on the impairment of social behaviors in adolescent mice exposed to social defeat stress as juveniles. From 1 day after the last stress exposure, the i.p. administration of vehicle (veh), desipramine 10 or 20 mg/kg (A1 and B1), sertraline 5 or 10 mg/kg (A2 and B2), aripiprazole 0.003 or 0.01 mg/kg (A3 and B3) or combined administration of sertraline 10 mg/kg with aripiprazole 0.01 mg/kg (A4 and B4) was started and continued once a day for 15 days. The social interaction test was performed 1 day and 15 days after the last stress exposure. Each column represents the mean±SEM (n=8–24). Two-way ANOVA. (A1) Fdefeat (1, 70)=94.7, P<.01; Fdrug (2, 70)=1.23, P=.30; Fdefeat × drug (1, 70)=0.51, P=.82. (A2) Fdefeat (1, 60)=5.28, P<.05; Fdrug (2, 60)=3.30, P<.05; Fdefeat × drug (1, 60)=3.77, P=.57. (A3) Fdefeat (1, 96)=33.3, P<.01; Fdrug (2, 96)=3.10, P<.05; Fdefeat × drug (1, 96)=0.17, P=.68. (A4) Fdefeat (1, 69)=9.83, P<.01; Fdrug (1, 69)=0.95, P=.33; Fdefeat × drug (1, 69)=0.57, P=.45. (B1) Fdefeat (1, 70)=13.1, P<.01; Fdrug (2, 70)=1.84, P=.17; Fdefeat × drug (1, 70)=0.17, P=.68. (B2) Fdefeat (1, 60) =4.58, P<.05; Fdrug (2, 60)=1.87, P=.16; Fdefeat × drug (1, 60)=1.48, P=.23. (B3) Fdefeat (1, 96)=18.6, P<.01; Fdrug (2, 96)=0.73, P=.49; Fdefeat × drug (1, 96)=1.34, P=.25. (B4) Fdefeat (1, 69)=6.80, P<.05; Fdrug (1, 69)=0.14, P=.71; Fdefeat × drug (1, 69)=10.1, P<.01. ##P<.01 vs vehicle-administrated control group, †P<.05 vs vehicle-administrated defeated group (Bonferroni’s test). control: mice exposed to an empty cage, defeated: mice exposed to an aggressive ICR mouse.
Acute administration of desipramine, sertraline, and aripiprazole did not affect the decrease in time spent in the interaction zone in the second session to expose an unfamiliar ICR mouse when tested 1 day after the last stress exposure (Figure 3A1–3). When tested 15 days after the last stress exposure, their repeated administration also did not affect body weight gain (data not shown) and the decrease in time spent in the interaction zone in the second session (Figure 3B1–3).
In the first session of acute administration, there was no significant difference among the groups in the time spent in the interaction zone (data not shown), whereas the repeated administration of 20 mg/kg desipramine and 0.003 and 0.01 mg/kg aripiprazole led to significantly increased time spent in the interaction zone in the first session compared with vehicle in the defeated group [2-way ANOVA; desipramine: Fdefeat (1, 70)=3.62, P=.06; Fdrug (2, 70)=1.11, P=.34; Fdefeat × drug (1, 70)=8.53, P<.01, sertraline: Fdefeat (1, 60)=2.00, P=.16; Fdrug (2, 60)=0.28, P=.76; Fdefeat × drug (1, 60)=7.34, P<.01, aripiprazole: Fdefeat (1, 96)=0.66, P=.42; Fdrug (2, 96)=2.53, P=.09; Fdefeat × drug (1, 96)=4.07, P<.05] (data not shown).
Additive Effect of Sertraline with Aripiprazole on the Impairment of Social Behaviors in Adolescent Mice Exposed to Social Defeat Stress as Juveniles
Both clinical and preclinical studies have demonstrated adjunctive use of aripiprazole as an augmentation strategy when combined with SSRIs (Kamei et al., 2008; Bourin et al., 2009). The present results shown in Figures 3A1–3 and 3B1–3 indicate exposure to social defeat stress as juveniles leads to the treatment-resistant impairment of social behaviors in adolescents, which is potentially caused by dysfunction of serotonergic and dopaminergic neuronal systems (Table 1; Figure 2). To investigate the effect of combined administration of sertraline and aripiprazole on the impairment of social behaviors, we used their ineffective doses, which were 10 mg/kg and 0.01 mg/kg, respectively (Figure 3A4 and B4).
In the second session, acute combined administration of sertraline with aripiprazole did not affect the impairment of social behaviors when tested 1 day after the last stress exposure, whereas repeated combined administration significantly attenuated it when tested 15 days after that (Figures 3A4 and B4). In the first session, however, there was no significant difference between groups in the time spent in the interaction zone when tested at 1 and 15 days after the last stress exposure, except for repeated combined administration in the defeated group at 15 days [2-way ANOVA; when tested 15 days: FDefeat (1, 69)=0.27, P=.60; FDrug (1, 69)=2.34, P=.13; FDefeat × Drug (1, 69)=0.16, P=.68, when tested 15 day: FDefeat (1, 69)=1.05, P=.31; FDrug (1, 69)=2.88, P=.09; FDefeat × Drug (1, 69)=3.86, P=.05] (data not shown).
Combined administration of sertraline and aripiprazole did not affect body weight gain (data not shown) and the exploratory activity (supplemental Figure S2).
Discussion
Adult rodents exposed to social defeat stress repeatedly display anxiety- and depressive-like behaviors (Kudryavtseva et al., 1991; Avgustinovich et al., 2005) such as the impairment of social behaviors (Berton et al., 2006; Tsankova et al., 2006), resembling the clinical symptoms of stress-related psychiatric disorders. In the present study, mice exposed to social defeat stress as juveniles showed the persistent impairment of social behaviors in adolescents, which is consistent with our previous finding (Mouri et al., 2018). The impairment of social behaviors was not due to motor dysfunction, since the mice showed no difference in locomotor activity in the first session of social interaction test regardless of their exposure to social defeat stress. The impairment of social behaviors was also replicated even when the C57BL/6J (same strain) mouse was present 1 day after last stress exposure (Mouri et al., 2018). Thus, it is unlikely that the defeated mice did not approach the unfamiliar target ICR mouse only because of the social fear response to the aggressor. On the other hand, social defeat stress exposure as juveniles did not induce pronounced anxiety-like behaviors (Mouri et al., 2018). The immobility in the forced swimming test was enhanced between 1 day and 4 weeks after the last stress, suggesting that vulnerability against intense stress was generated by repeated exposure to juvenile stress (Mouri et al., 2018). Therefore, juvenile rodents exposed to social defeat stress repeatedly display depressive-like but not anxiety-like behaviors, and the impairment of social behaviors by social defeat stress might be caused by decrease of sociality rather than anxiety to the target mouse. In addition, the present stressed mouse model may be useful as a juvenile stress-based experimental model for the study of psychiatric disorders in adolescents.
The detailed mechanisms of social behavioral changes in this model have not been elucidated yet. However, it has been suggested that monoaminergic neuronal systems are important modulators of the responses to stress (Torres et al., 2002). Continued or chronic stress exposure is found to have a negative influence on the serotonergic neuronal system and may increase the 5-HT sensitivity or vulnerability as a compensatory response (Firk and Markus, 2007). In this model, the 5-HT metabolism in the PFC and AMG of the nontested control group were increased following the social interaction test, whereas there were no changes in NA and DA metabolisms. Interestingly, an increase in the utilization of 5-HT was not observed following the social interaction test in mice exposed to the stress. Thus, these findings suggest that the serotonergic neuronal dysfunction is involved in the expression of impaired social behaviors. Meanwhile, the utilization of not only 5-HT but also DA in the NAc, HIP, and AMG was decreased in adolescent mice exposed to social defeat stress as juveniles following the social interaction test. Namely, no change in DA metabolism of the tested control group was observed, while the metabolism was significantly decreased on social defeat stress exposure in the tested mice (the tested defeated group) compared with that in the tested control group. Stress-induced inhibition of DA release in the NAc can explain the impaired defensive reactions under aversive conditions observed following stressful experiences (Cabib and Puglisi-Allegra, 1996). Taken together with these reports (Cabib and Puglisi-Allegra, 1996; Firk and Markus, 2007), our findings suggest that the not only serotonergic, but also dopaminergic neuronal systems in adolescent mice are impaired by social defeat stress exposure as juveniles. There are functional changes associated with the decreased the utilization of 5-HT and DA, which might be involved in the impairment of social behaviors. This could be a model of decreased interest as the symptom of depression.
The utilization of DA was increased in the PFC and NAc of adolescent mice exposed to social defeat stress (Tanaka et al., 2012). The reason for this discrepancy is unknown but may be because of difference in aging between samples exposed to social defeat stress at 3 weeks (juvenile) and 6 weeks (adolescent). Prominent developmental transformations are seen in the PFC and limbic regions of the brain of adolescents across a variety of species; alterations include an apparent shift in the balance between the mesocortical and mesolimbic dopaminergic neuronal system (Spear, 2000). Different development transformations in mesocortical and mesolimbic dopaminergic neuronal systems between juvenile and adolescent mice exposed to social defeat stress may be involved.
Acute or repeated administration of traditional antidepressants have been shown to be effective in many animal models of MDD (Caldarone et al., 2015). Repeated administration of paroxetine, which is used widely in humans, attenuated impairment of social interaction in adult, defeated mice (Xu et al., 2018). In the present study, acute and repeated administration of desipramine and sertraline did not attenuate the persistent impairment of social behaviors in adolescent mice exposed to social defeat stress as juveniles. Our findings suggest that exposure to social defeat stress as juveniles induces an antidepressant-resistant impairment of social behaviors in adolescents. Antidepressants are widely used in the treatment of depression in adolescents, although there are antidepressant-resistant in adolescents (Cipriani et al., 2016). Augmentation pharmacotherapy refers to the addition of drugs that are not standard antidepressants to enhance the effect of a classical antidepressant drug (Carvalho et al., 2007). Clinically, antidepressant activity of antipsychotics has been observed when administered either alone or in combination with an antidepressant (Kamei et al., 2008). DA-D2 and D3 receptor agonists have been tested as augmenting agents in antidepressant-resistant forms of MDD (Carvalho et al., 2007). Aripiprazole is an atypical antipsychotic with a novel pharmacological profile, acting as a partial DA-D2 and D3 receptor agonist (Bourin et al., 2009). In the present study, however, acute and repeated administration of aripiprazole itself did not attenuate the persistent impairment of social behaviors in adolescent mice exposed to social defeat stress as juveniles despite the DA hypofunction observed in the defeated mice. The functional changes in the utilization of 5-HT and DA were observed in adolescent mice exposed to social defeat stress as juveniles. These results indicate that multiple functional changes in monoaminergic neuronal systems are involved in the treatment-resistant impairment of social behaviors in adolescent mice exposed to social defeat stress as juveniles. Treatment with a combination of atypical antipsychotic drugs and SSRIs had antidepressant effects in patients who did not respond to SSRI monotherapy (Kamei et al., 2008). Previous studies have indicated that aripiprazole combined with inactive doses of antidepressants attenuated depressive-like behaviors in a forced swim test (Bourin et al., 2009). At noneffective doses individually, repeated administration of a combination of sertraline and aripiprazole showed additive effects without affecting body weight gain and the exploratory activity on the impairment of social behaviors in adolescent mice exposed to social defeat stress as juveniles in the present study. Thus, the combined administration of sertraline with aripiprazole may be useful as a new treatment strategy for treatment-resistant of stress stress-related psychiatric disorders.
It is unclear why repeated but not acute administration of a combination of sertraline and aripiprazole attenuates the impairment of social behaviors induced by social defeat stress exposure as juveniles, whereas the serotonergic and dopaminergic neuronal systems were impaired 1 day after the last exposure to social defeat stress. There are only a few clinical or preclinical studies that have assessed the effects of chronic administration of aripiprazole in combination with antidepressants in adolescent depressed patients or in mice. We found changes in the utilizations of the 5-HT (P<.05), and DA (P=.08), NA (P=.06) in the AMG (supplemental Figure S1) but not in other brain regions (PFC, NAs, and HIP: data not shown) 15 days after the last social defeat stress exposure, indicating monoaminergic neuronal dysfunction in the AMG. Multiple functional changes in the monoaminergic neuronal systems of the AMG occurred over the 15 days and led to persistent impairment of the social behaviors in the adolescent mice. Repeated administration of a combination of sertraline and aripiprazole prevented the development of multiple functional changes in monoaminergic neuronal systems, leading to reversal of social impairment. Emotionally related learning, including stress exposure events, is mediated through the interactions of the basolateral HIP and AMG formation (Benes, 2010). We reported that the stress exposure induced the persistent impairment of social behaviors associated with suppression of the hippocampal neurogenesis (Mouri et al., 2018). In the present study, we found the changes in the utilizations of the 5-HT and DA in the AMG 15 days after the last social defeat stress exposure (supplemental Figure S1). Thus, repeated administration of a combination of sertraline and aripiprazole may improve monoaminergic neuronal dysfunctions in the HIP and/or AMG 1 and/or 15 days after the stress exposure. Further studies are needed to investigate the effect of social defeat stress exposure in monoaminergic neuronal systems of the HIP-AMG pathways and morphological changes, such as spine densities and dendritic lengths, during development of monoaminergic neuronal systems (Lyttle et al., 2015; Suri et al., 2015).
In the present study, there were no significant changes in NA metabolism in the all brain regions of the mice exposed to social defeat stress when the all groups were compared. Thus, it is unlikely that the impairment of social behaviors is due to changes in the noradrenergic neuronal system relative to that of the controls, although the noradrenergic neuronal system has been explicitly implicated in pathophysiological conditions induced by the stress exposure (Glavin, 1985). Further, previous studies have shown that NA transporter-knockout mice are resistant to the impairment of social behaviors induced by social defeat stress exposure (Haenisch et al., 2009). It remains unclear whether noradrenergic neuronal systems are involved in the treatment-resistant impairment of social behaviors in adolescent mice exposed to social defeat stress as juveniles. The mechanisms of additive effect also remain unclear and might be mediated by multiple receptor properties and other neuronal systems including the glutamatergic and cholinergic neuronal systems. Aripiprazole acts as not only a partial agonist at the DA-D2 and D3 receptors but also as a partial agonist at the serotonin 5-HT1A receptors and an antagonist at the serotonin receptors (Bourin et al., 2009). Both monoaminergic and glutamatergic (Sanacora et al., 2012) as well as cholinergic (Mineur et al., 2013) neuronal systems have driven the research on the impairment of social behaviors induced by social defeat stress exposure. The additive effect may be mediated, at least partly, by the activation of the serotonergic and dopaminergic neuronal systems. Further studies are needed to investigate the complex mechanisms behind the functional changes in both the noradrenergic and serotonergic/dopaminergic neuronal systems, including receptor functions and other neuronal systems, leading to the development of treatment-resistant impairment of social behaviors.
Aripiprazole is a dopaminergic neuronal system stabilizer; namely, a drug that can enhance dopaminergic neuronal activity when it is diminished or suppress it when it is increased (Stahl, 2001a, 2001b). Bourin et al. (2009) referred that the combination of aripiprazole and selective serotonin reuptake inhibitors (SSRIs) in outpatients with depression who did not experience significant clinical improvement after taking antidepressants alone was performed in clinical trials (Papakostas et al., 2004; Simon and Nemeroff, 2005; Marcus et al., 2008). These findings suggest that aripiprazole adjunction could be used as an augmentation strategy when combined with SSRIs (Bourin et al., 2009). Bourin et al (2009) demonstrated that combined administration of subactive doses of antidepressants (which activate the serotonergic system) with aripiprazole (which activates the dopaminergic system) induced an antidepressive-like effect in a mouse model of depression. Thus, we speculated that aripiprazole may work as an agonistic rather than antagonistic for dopamine-D2 receptor in the present experiment.
In conclusion, exposure to social defeat stress as juveniles induces the treatment-resistant impairment of social behaviors in adolescents through the functional changes in the serotonergic and dopaminergic neuronal systems. Administration of a combination of sertraline and aripiprazole may be useful as a new treatment strategy in adolescents, who had been exposed to adverse juvenile experiences, with treatment-resistant stress-related psychiatric disorders.
Funding
This study was supported by Grants-in-Aid for Scientific Research C (grant nos. 24590219, 26460240, 16K08421), B (grant no. 17H04252), and the private University Research Project from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, Grant-in-Aid for the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and development, AMED, The Adaptable and Seamless Technology Transfer Program Through Target-driven R&D, Japan Science and Technology Agency (grant no. AS251Z03018), Meijo University Research Institute grant, and Smoking Research Foundation Grant for Biomedical Research.
Statement of Interest
Dr. Norio Ozaki has received research support or speakers’ honoraria from, or has served as a consultant to Astellas, Dainippon Sumitomo, Eisai, Eli Lilly, Glaxo Smith Kline, Janssen, Meiji Seika Pharma, Mochida, MSD, Nihon Medi-Physics, Novartis, Ono, Otsuka, Pfizer, Shionogi, Takeda, Taisho, Tanabe Mitsubishi, Teijin, Tsumura, and Yoshitomi outside the submitted work. Dr. Yukihiro Noda has also received research support and speakers’ honoraria from Sumitomo Dainippon Pharma, Janssen Pharmaceuticals, Otsuka Pharmaceutical, and Kyorin Pharmaceutical outside the submitted work.
Supplementary Material
Acknowledgments
We thank Mayu Ukai, Masayuki Taniguchi, and all staff members of Faculty of Pharmacy, Meijo University that were involved in this study.
References
- Afifi TO.(2011)Child maltreatment in Canada: an understudied public health problem. Can J Public Health 102:459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annerbäck EM, Sahlqvist L, Svedin CG, Wingren G, Gustafsson PA(2012)Child physical abuse and concurrence of other types of child abuse in Sweden-associations with health and risk behaviors. Child Abuse Negl 36:585–595. [DOI] [PubMed] [Google Scholar]
- Avgustinovich DF, Kovalenko IL, Kudryavtseva NN(2005)A model of anxious depression: persistence of behavioral pathology. Neurosci Behav Physiol 35:917–924. [DOI] [PubMed] [Google Scholar]
- Benes FM.(2010)Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharmacology 35:239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ(2006)Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868. [DOI] [PubMed] [Google Scholar]
- Bourin M, Chenu F, Prica C, Hascoët M(2009)Augmentation effect of combination therapy of aripiprazole and antidepressants on forced swimming test in mice. Psychopharmacology (Berl) 206:97–107. [DOI] [PubMed] [Google Scholar]
- Boyarskikh UA, Bondar NP, Filipenko ML, Kudryavtseva NN(2013)Downregulation of serotonergic gene expression in the Raphe nuclei of the midbrain under chronic social defeat stress in male mice. Mol Neurobiol 48:13–21. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S(1996)Stress, depression and the mesolimbic dopamine system. Psychopharmacology (Berl) 128:331–342. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Zachariou V, King SL(2015)Rodent models of treatment-resistant depression. Eur J Pharmacol 753:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JL, Covington HE 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han MH(2010)Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci 30:16453–16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Cavalcante JL, Castelo MS, Lima MC(2007)Augmentation strategies for treatment-resistant depression: a literature review. J Clin Pharm Ther 32:415–428. [DOI] [PubMed] [Google Scholar]
- Chen P, Fan Y, Li Y, Sun Z, Bissette G, Zhu MY(2012)Chronic social defeat up-regulates expression of norepinephrine transporter in rat brains. Neurochem Int 60:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, Coghill D, Zhang Y, Hazell P, Leucht S, Cuijpers P, Pu J, Cohen D, Ravindran AV, Liu Y, Michael KD, Yang L, Liu L, Xie P(2016)Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet 388:881–890. [DOI] [PubMed] [Google Scholar]
- Firk C, Markus CR(2007)Review: serotonin by stress interaction: a susceptibility factor for the development of depression? J Psychopharmacol 21:538–544. [DOI] [PubMed] [Google Scholar]
- Flügge G.(2000)Regulation of monoamine receptors in the brain: dynamic changes during stress. Int Rev Cytol 195:145–213. [DOI] [PubMed] [Google Scholar]
- Glavin GB.(1985)Stress and brain noradrenaline: a review. Neurosci Biobehav Rev 9:233–243. [DOI] [PubMed] [Google Scholar]
- Haenisch B, Bilkei-Gorzo A, Caron MG, Bönisch H(2009)Knockout of the norepinephrine transporter and pharmacologically diverse antidepressants prevent behavioral and brain neurotrophin alterations in two chronic stress models of depression. J Neurochem 111:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei J, Miyata S, Sunohara T, Kamei A, Shimada M, Ohsawa M(2008)Potentiation of the antidepressant-like effect of fluoxetine by aripiprazole in the mouse tail suspension test. J Pharmacol Sci 108:381–384. [DOI] [PubMed] [Google Scholar]
- Krishnan V, et al. . 2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Neumeister A(2009)Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res 1293:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA(1991)Social model of depression in mice of C57bl/6J strain. Pharmacol Biochem Behav 38:315–320. [DOI] [PubMed] [Google Scholar]
- Lyttle K, Ohmura Y, Konno K, Yoshida T, Izumi T, Watanabe M, Yoshioka M(2015)Repeated fluvoxamine treatment recovers juvenile stress-induced morphological changes and depressive-like behavior in rats. Brain Res 1616:88–100. [DOI] [PubMed] [Google Scholar]
- Marcus RN, McQuade RD, Carson WH, Hennicken D, Fava M, Simon JS, Trivedi MH, Thase ME, Berman RM(2008)The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 28:156–165. [DOI] [PubMed] [Google Scholar]
- McKenzie K, Scott DA(2012)Quantity of documentation of maltreatment risk factors in injury-related paediatric hospitalisations. BMC Public Health 12:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC(2010)Childhood adversities and adult psychopathology in the National Comorbidity Survey Replication (NCS-R) III: associations with functional impairment related to DSM-IV disorders. Psychol Med 40:847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR(2013)Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A 110:3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Frazer A(2004)Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol 7:193–218. [DOI] [PubMed] [Google Scholar]
- Mouri A, Sasaki A, Watanabe K, Sogawa C, Kitayama S, Mamiya T, Miyamoto Y, Yamada K, Noda Y, Nabeshima T(2012)MAGE-D1 regulates expression of depression-like behavior through serotonin transporter ubiquitylation. J Neurosci 32:4562–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri A, Ukai M, Uchida M, Hasegawa S, Taniguchi M, Ito T, Hida H, Yoshimi A, Yamada K, Kunimoto S, Ozaki N, Nabeshima T, Noda Y(2018)Juvenile social defeat stress exposure persistently impairs social behaviors and neurogenesis. Neuropharmacology 133:23–37. [DOI] [PubMed] [Google Scholar]
- Nagai T, Murai R, Matsui K, Kamei H, Noda Y, Furukawa H, Nabeshima T(2009)Aripiprazole ameliorates phencyclidine-induced impairment of recognition memory through dopamine D1 and serotonin 5-HT1a receptors. Psychopharmacology (Berl) 202:315–328. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Katsu H, Ando T, Nakajo R(2003)Possible alteration of tryptophan metabolism following repeated administration of sertraline in the rat brain. Brain Res Bull 59:293–297. [DOI] [PubMed] [Google Scholar]
- Noda Y, Mamiya T, Furukawa H, Nabeshima T(1997)Effects of antidepressants on phencyclidine-induced enhancement of immobility in a forced swimming test in mice. Eur J Pharmacol 324:135–140. [DOI] [PubMed] [Google Scholar]
- Noda Y, Miyamoto Y, Mamiya T, Kamei H, Furukawa H, Nabeshima T(1998)Involvement of dopaminergic system in phencyclidine-induced place preference in mice pretreated with phencyclidine repeatedly. J Pharmacol ExP Ther 286:44–51. [PubMed] [Google Scholar]
- Papakostas GI, Petersen TJ, Nierenberg AA, Murakami JL, Alpert JE, Rosenbaum JF, Fava M(2004)Ziprasidone augmentation of selective serotonin reuptake inhibitors (ssris) for SSRI-resistant major depressive disorder. J Clin Psychiatry 65:217–221. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ(2004)The mouse brain in stereotaxic coordinates. Compact 2nd ed Amsterdam, Boston: Elsevier Academic Press. [Google Scholar]
- Pittenger C, Duman RS(2008)Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33:88–109. [DOI] [PubMed] [Google Scholar]
- Placidi GP, Oquendo MA, Malone KM, Huang YY, Ellis SP, Mann JJ(2001)Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol Psychiatry 50:783–791. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M(2012)Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Haller J(2015)Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci 16:290–304. [DOI] [PubMed] [Google Scholar]
- Simon JS, Nemeroff CB(2005)Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry 66:1216–1220. [DOI] [PubMed] [Google Scholar]
- Spear LP.(2000)The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463. [DOI] [PubMed] [Google Scholar]
- Stahl SM.(2001a)Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 1, “Goldilocks” actions at dopamine receptors. J Clin Psychiatry 62:841–842. [DOI] [PubMed] [Google Scholar]
- Stahl SM.(2001b)Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 2: illustrating their mechanism of action. J Clin Psychiatry 62:923–924. [DOI] [PubMed] [Google Scholar]
- Steckler T, Risbrough V(2012)Pharmacological treatment of PTSD - established and new approaches. Neuropharmacology 62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri D, Teixeira CM, Cagliostro MK, Mahadevia D, Ansorge MS(2015)Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology 40:88–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Furuyashiki T, Kitaoka S, Senzai Y, Imoto Y, Segi-Nishida E, Deguchi Y, Breyer RM, Breyer MD, Narumiya S(2012)Prostaglandin E2-mediated attenuation of mesocortical dopaminergic pathway is critical for susceptibility to repeated social defeat stress in mice. J Neurosci 32:4319–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres IL, Gamaro GD, Vasconcellos AP, Silveira R, Dalmaz C(2002)Effects of chronic restraint stress on feeding behavior and on monoamine levels in different brain structures in rats. Neurochem Res 27:519–525. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ(2006)Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9:519–525. [DOI] [PubMed] [Google Scholar]
- Weber K, Rockstroh B, Borgelt J, Awiszus B, Popov T, Hoffmann K, Schonauer K, Watzl H, Pröpster K(2008)Stress load during childhood affects psychopathology in psychiatric patients. BMC Psychiatry 8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weich S, Patterson J, Shaw R, Stewart-Brown S(2009)Family relationships in childhood and common psychiatric disorders in later life: systematic review of prospective studies. Br J Psychiatry 194:392–398. [DOI] [PubMed] [Google Scholar]
- Xu D, Sun Y, Wang C, Wang H, Wang Y, Zhao W, Bao G, Wang F, Cui Z, Jiang B(2018)Hippocampal mTOR signaling is required for the antidepressant effects of paroxetine. Neuropharmacology 128:181–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.